Catalog No.
KDA29104
Description
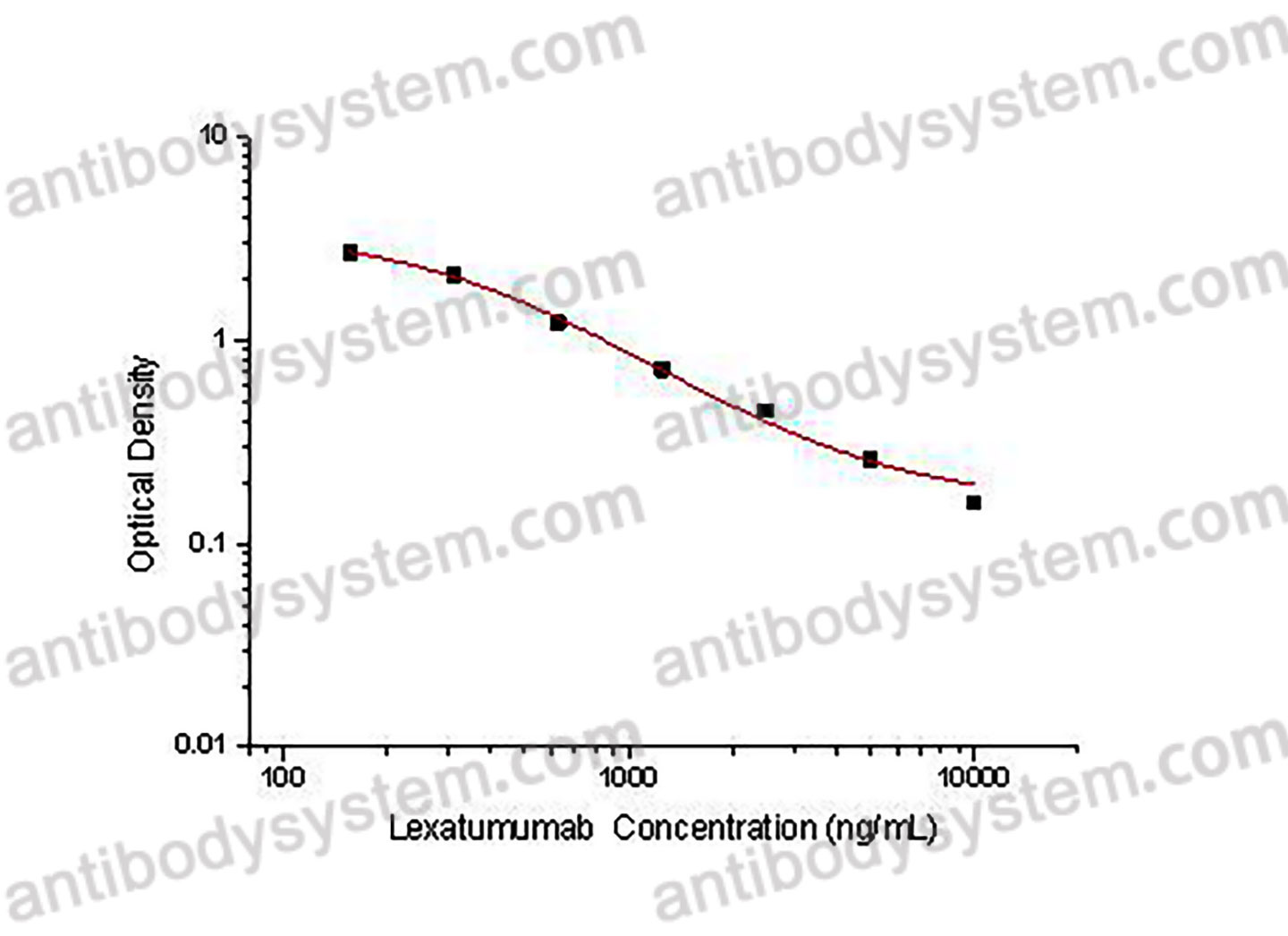
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD262 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Lexatumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Lexatumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Lexatumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Lexatumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
120.42 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
5764.3
|
1313.3
|
334.2
|
5327.5
|
1213.6
|
269.8
|
|
Standard deviation
|
316.3
|
102.3
|
43.9
|
339.1
|
55.6
|
29.4
|
|
CV (%)
|
5.5
|
7.8
|
13.1
|
6.4
|
4.6
|
10.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
HGS-ETR2, CAS: 845816-02-6
Background
Drozitumab is a human monoclonal antibody directed against tumor necrosis factor receptor superfamily member 10B (TNFRSF10B), a member of tumor necrosis factor related apoptosis-inducing ligand (TRAIL). It was developed by Genentech as a drug for the treatment of cancers. This drug has been investigating in various trials for treating cancers including chondrosarcoma, colorectal cancer, non-Hodgkin lymphoma, and non-small cell lung cancer. The efficacy and selectivity of drozitumab in rhabdomyosarcoma (RMS) has been evaluated in preclinical models. And results showed that drozitumab is effective, in vitro, against the majority of RMS cell lines that express caspase-8 and, in vivo, may provide long-term control of RMS. In preclinical glioblastoma models, drozitumab has been found to induce apoptosis and colony formation in several glioblastoma cell lines combined with BV6 (combination index<0.1). A study about triple-negative breast cancer reported that drozitumab could induce apoptosis in mesenchymal TNBC cell lines but not in cell lines from other breast cancer subtypes. The previous study about the treatment of pancreatic ductal adenocarcinoma has revealed that drozitumab could selectively eliminate CSCs, resulting in tumor growth inhibition and even regression of pancreatic tumors.
Carboxypeptidase A4 negatively regulates HGS-ETR1/2-induced pyroptosis by forming a positive feedback loop with the AKT signalling pathway., PMID:38049405
Celastrol enhances TRAIL‑R2‑mediated apoptosis and cytotoxicity in human renal cell carcinoma cells in caspase‑dependent manner., PMID:37997857
TRAIL-mediated signaling in bladder cancer: realization of clinical efficacy of TRAIL-based therapeutics in medical oncology., PMID:37432489
miR-3132 upregulates surface TRAIL to induce apoptotic cell death in cancer cells., PMID:35141020
A patch of positively charged residues regulates the efficacy of clinical DR5 antibodies in solid tumors., PMID:34731630
Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells., PMID:30947287
Focal adhesion kinase inhibitor PF573228 and death receptor 5 agonist lexatumumab synergistically induce apoptosis in pancreatic carcinoma., PMID:28459212
Methionine Deprivation Induces a Targetable Vulnerability in Triple-Negative Breast Cancer Cells by Enhancing TRAIL Receptor-2 Expression., PMID:25724522
Small molecule inhibitor YM155-mediated activation of death receptor 5 is crucial for chemotherapy-induced apoptosis in pancreatic carcinoma., PMID:25344582
Blocks to thyroid cancer cell apoptosis can be overcome by inhibition of the MAPK and PI3K/AKT pathways., PMID:24603332
Sorafenib sensitizes solid tumors to Apo2L/TRAIL and Apo2L/TRAIL receptor agonist antibodies by the Jak2-Stat3-Mcl1 axis., PMID:24086526
Death receptors as targets in cancer., PMID:23638798
TRAIL-based therapeutic approaches for the treatment of pediatric malignancies., PMID:23458616
Low-dose anisomycin sensitizes melanoma cells to TRAIL induced apoptosis., PMID:23192275
Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors., PMID:23071222
Dacarbazine and the agonistic TRAIL receptor-2 antibody lexatumumab induce synergistic anticancer effects in melanoma., PMID:23029050
RIP1 protein-dependent assembly of a cytosolic cell death complex is required for inhibitor of apoptosis (IAP) inhibitor-mediated sensitization to lexatumumab-induced apoptosis., PMID:22927431
Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: evidence for the role of c-Jun N-terminal kinase activation., PMID:22895172
RIP1 is required for IAP inhibitor-mediated sensitization for TRAIL-induced apoptosis via a RIP1/FADD/caspase-8 cell death complex., PMID:22890322
Therapeutic targeting of the TNF superfamily: a promising treatment for advanced endometrial adenocarcinoma., PMID:22885470
Cell death via DR5, but not DR4, is regulated by p53 in myeloma cells., PMID:22738917
Synthetic retinoid CD437 induces apoptosis and acts synergistically with TRAIL receptor-2 agonist in malignant melanoma., PMID:22446330
Delineation of apoptotic genes for synergistic apoptosis of lexatumumab and anthracyclines in human renal cell carcinoma cells by polymerase chain reaction array., PMID:22205156
Killing of resistant cancer cells with low Bak by a combination of an antimesothelin immunotoxin and a TRAIL Receptor 2 agonist antibody., PMID:21813632
Prediction of proapoptotic anticancer therapeutic response in vivo based on cell death visualization and TRAIL death ligand-receptor interaction., PMID:21785270
Spectral imaging-based methods for quantifying autophagy and apoptosis., PMID:21757995
Death receptor 4 is preferentially recruited to lipid rafts in chronic lymphocytic leukemia cells contributing to tumor necrosis related apoptosis inducing ligand-induced synergistic apoptotic responses., PMID:21699383
Off-target lapatinib activity sensitizes colon cancer cells through TRAIL death receptor up-regulation., PMID:21653830
Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer., PMID:21653692
Reactive oxygen species is essential for cycloheximide to sensitize lexatumumab-induced apoptosis in hepatocellular carcinoma cells., PMID:21347335
High TRAIL-R3 expression on leukemic blasts is associated with poor outcome and induces apoptosis-resistance which can be overcome by targeting TRAIL-R2., PMID:21281967
TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors., PMID:20940278
Gateways to clinical trials., PMID:20664824
Aldehyde dehydrogenase (ALDH) activity does not select for cells with enhanced aggressive properties in malignant melanoma., PMID:20505780
TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5., PMID:20354842
Primary ovarian cancer cells are sensitive to the proaptotic effects of proteasome inhibitors., PMID:20126991
Combined treatment with lexatumumab and irradiation leads to strongly increased long term tumour control under normoxic and hypoxic conditions., PMID:19860913
Altered regulation of extrinsic apoptosis pathway in HCV-infected HCC cells enhances susceptibility to mapatumumab-induced apoptosis., PMID:19788693
Phosphorothioate-modified TLR9 ligands protect cancer cells against TRAIL-induced apoptosis., PMID:19734228
Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors., PMID:19633048
Efficacy of triple therapies including ionising radiation, agonistic TRAIL antibodies and cisplatin., PMID:19424623
A double hit to kill tumor and endothelial cells by TRAIL and antiangiogenic 3TSR., PMID:19366809
Enhancement of lexatumumab-induced apoptosis in human solid cancer cells by Cisplatin in caspase-dependent manner., PMID:19276256
Efficacy of a triple treatment with irradiation, agonistic TRAIL receptor antibodies and EGFR blockade., PMID:19224142
Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib., PMID:19174554
Aspirin sensitizes cancer cells to TRAIL-induced apoptosis by reducing survivin levels., PMID:18483385