Catalog No.
DHC83402
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4
Clonality
Monoclonal
Target
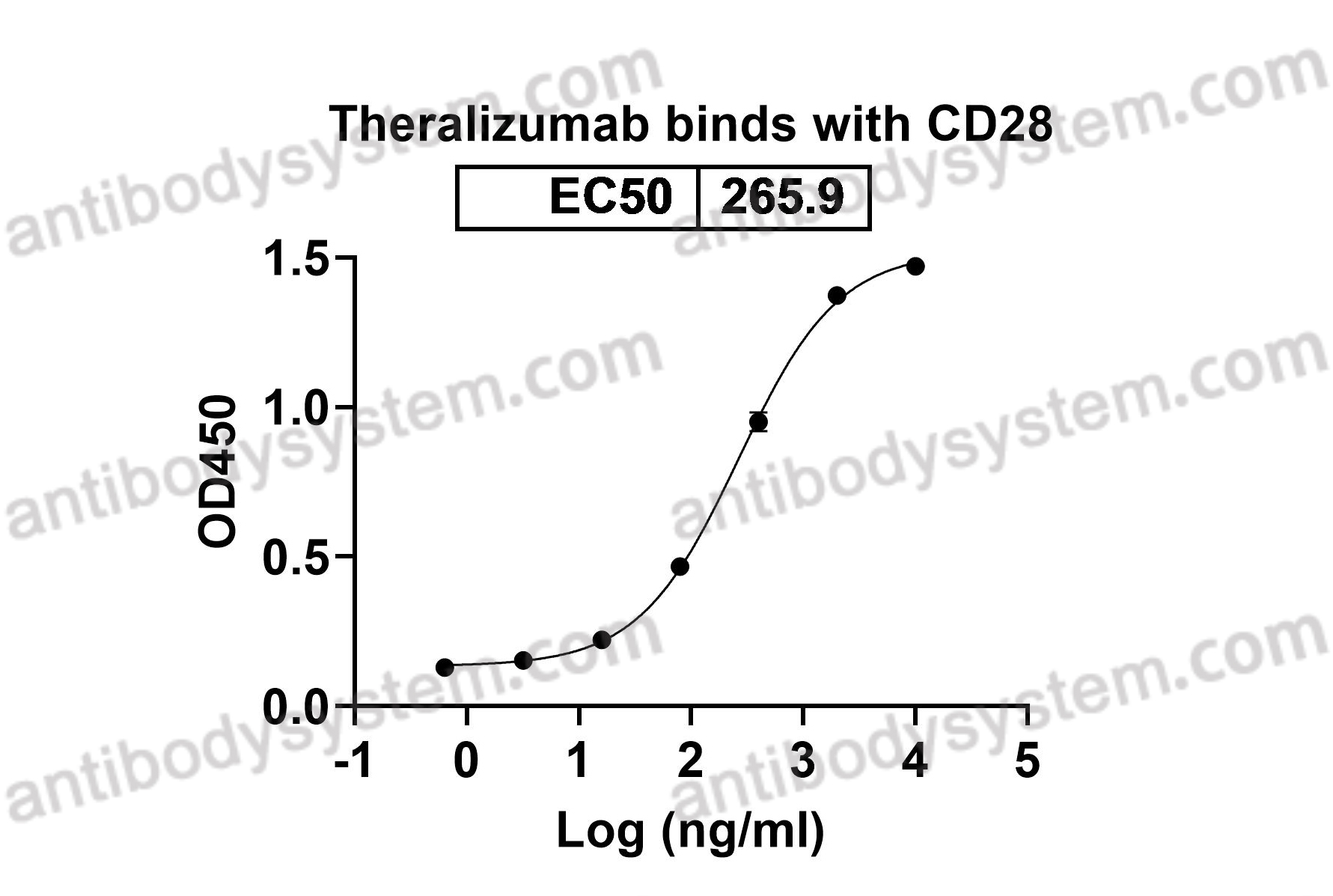
CD28, TP44, T-cell-specific surface glycoprotein CD28
Concentration
2.82 mg/ml
Endotoxin level
Please contact with the lab for this information.
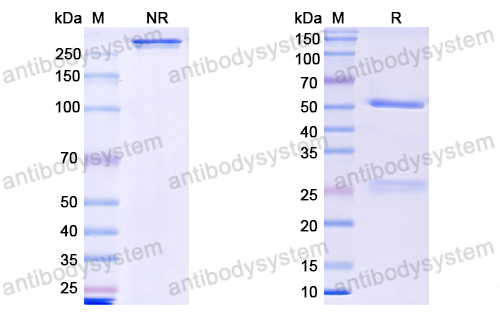
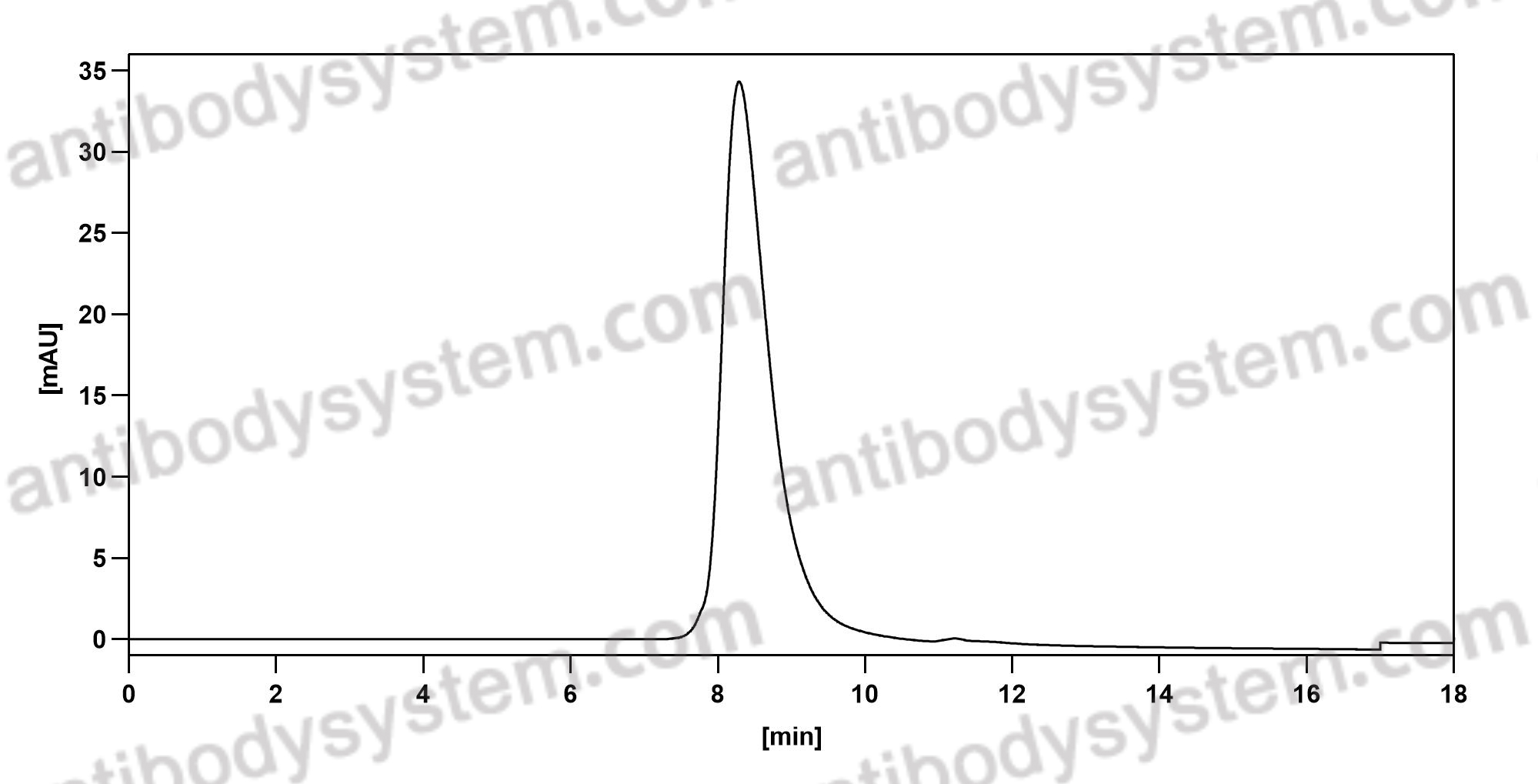
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P10747
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
TGN1412, TAB08, TAB-08, CD28-SuperMAB,CAS: 906068-56-2
Clone ID
Theralizumab
Mutation of the TGN1412 anti-CD28 monoclonal antibody lower hinge confers specific FcγRIIb binding and retention of super-agonist activity., PMID:36997299
CD28 Superagonist Shock and Blockage of Motogenic T Cell Cascade., PMID:33968078
Regulatory and strategic considerations for addressing immunogenicity and related responses in biopharmaceutical development programs., PMID:33948231
Development of the first reference antibody panel for qualification and validation of cytokine release assay platforms - Report of an international collaborative study., PMID:33458650
The blood endothelial cell chamber - An innovative system to study immune responses in drug development., PMID:33310662
Patients with gastrointestinal irritability after TGN1412-induced cytokine storm displayed selective expansion of gut-homing αβ and γδT cells., PMID:33048222
Immune reconstitution and clinical recovery following anti-CD28 antibody (TGN1412)-induced cytokine storm., PMID:33033851
Myelopoiesis of acute inflammation: lessons from TGN1412-induced cytokine storm., PMID:32862238
First-in-human dose: current status review for better future perspectives., PMID:32488334
Human Fcγ receptors compete for TGN1412 binding that determines the antibody's effector function., PMID:31002172
Evaluation of a TGN1412 analogue using in vitro assays and two immune humanized mouse models., PMID:30914376
Development of transplant immunosuppressive agents - considerations in the use of animal models., PMID:30332905
Different players generate positive responses in two in vitro cytokine assay formats with aqueous and immobilized TGN1412 analog., PMID:29787754
TGN-1412 and BIA-2474 Trials with Tragic End: Lessons Learnt To Make Clinical Trials Safer., PMID:29779485
An Autocrine TNFα-Tumor Necrosis Factor Receptor 2 Loop Promotes Epigenetic Effects Inducing Human Treg Stability In Vitro., PMID:29619032
A call to incorporate systems theory and human factors into the existing investigation of harm in clinical research involving healthcare products., PMID:28681444
Animal extrapolation in preclinical studies: An analysis of the tragic case of TGN1412., PMID:28039775
From TGN1412 to TAB08: the return of CD28 superagonist therapy to clinical development for the treatment of rheumatoid arthritis., PMID:27586803
Is an in vitro whole blood cytokine assay useful to detect the potential risk of severe infusion reaction of monoclonal antibody pharmaceuticals?, PMID:27432238
The rise and fall of the CD28 superagonist TGN1412 and its return as TAB08: a personal account., PMID:27191544
TGN1412 Induces Lymphopenia and Human Cytokine Release in a Humanized Mouse Model., PMID:26959227
Cytokine release assays for the prediction of therapeutic mAb safety in first-in man trials--Whole blood cytokine release assays are poorly predictive for TGN1412 cytokine storm., PMID:25960173
Experimental drug that injured UK volunteers resumes in human trials., PMID:25838401
An autologous endothelial cell:peripheral blood mononuclear cell assay that detects cytokine storm responses to biologics., PMID:25746794
The return of the prodigal son and the extraordinary development route of antibody TGN1412 - lessons for drug development and clinical pharmacology., PMID:25711949
Failure to upregulate cell surface PD-1 is associated with dysregulated stimulation of T cells by TGN1412-like CD28 superagonist., PMID:25517314
Upregulation of FcγRIIb on monocytes is necessary to promote the superagonist activity of TGN1412., PMID:25395427
CD28 signals the differential control of regulatory T cells and effector T cells., PMID:24652756
Advantages of Papio anubis for preclinical testing of immunotoxicity of candidate therapeutic antagonist antibodies targeting CD28., PMID:24598534
Cell contact-dependent priming and Fc interaction with CD32+ immune cells contribute to the TGN1412-triggered cytokine response., PMID:24470499
Cytokine release assays: current practices and future directions., PMID:24412476
Human regulatory T cells are selectively activated by low-dose application of the CD28 superagonist TGN1412/TAB08., PMID:24374661
Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release., PMID:23701319
Giving monoclonal antibodies to healthy volunteers in phase 1 trials: is it safe?, PMID:23438102
A simple whole blood bioassay detects cytokine responses to anti-CD28SA and anti-CD52 antibodies., PMID:23280407
Antibody C region influences TGN1412-like functional activity in vitro., PMID:23150712
Dynamics of a cytokine storm., PMID:23049677
A pharmacologic perspective on newly emerging T-cell manipulation technologies., PMID:23039307
A whole blood in vitro cytokine release assay with aqueous monoclonal antibody presentation for the prediction of therapeutic protein induced cytokine release syndrome in humans., PMID:22986013
After TGN1412: recent developments in cytokine release assays., PMID:22967038
ICOS-LICOS interaction is critically involved in TGN1412-mediated T-cell activation., PMID:22577174
The storm has cleared: lessons from the CD28 superagonist TGN1412 trial., PMID:22487653
Predicting cytokine storms: it's about density., PMID:22194391
In vitro cytokine release assays: reducing the risk of adverse events in man., PMID:22136053
A predictive biomimetic model of cytokine release induced by TGN1412 and other therapeutic monoclonal antibodies., PMID:22074378
Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412., PMID:21931118
Safety assessment of immunomodulatory biologics: the promise and challenges of regulatory T-cell modulation., PMID:21913866
Ipilimumab (Yervoy) and the TGN1412 catastrophe., PMID:21821307
Endothelial cells co-stimulate peripheral blood mononuclear cell responses to monoclonal antibody TGN1412 in culture., PMID:21493088
Were monocytes responsible for initiating the cytokine storm in the TGN1412 clinical trial tragedy?, PMID:20964641