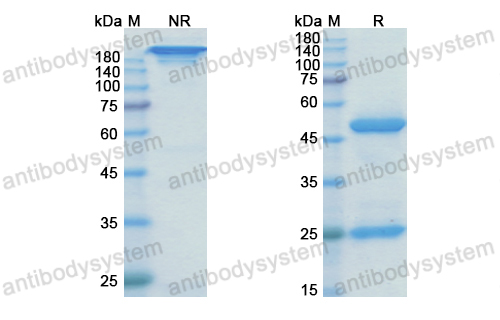
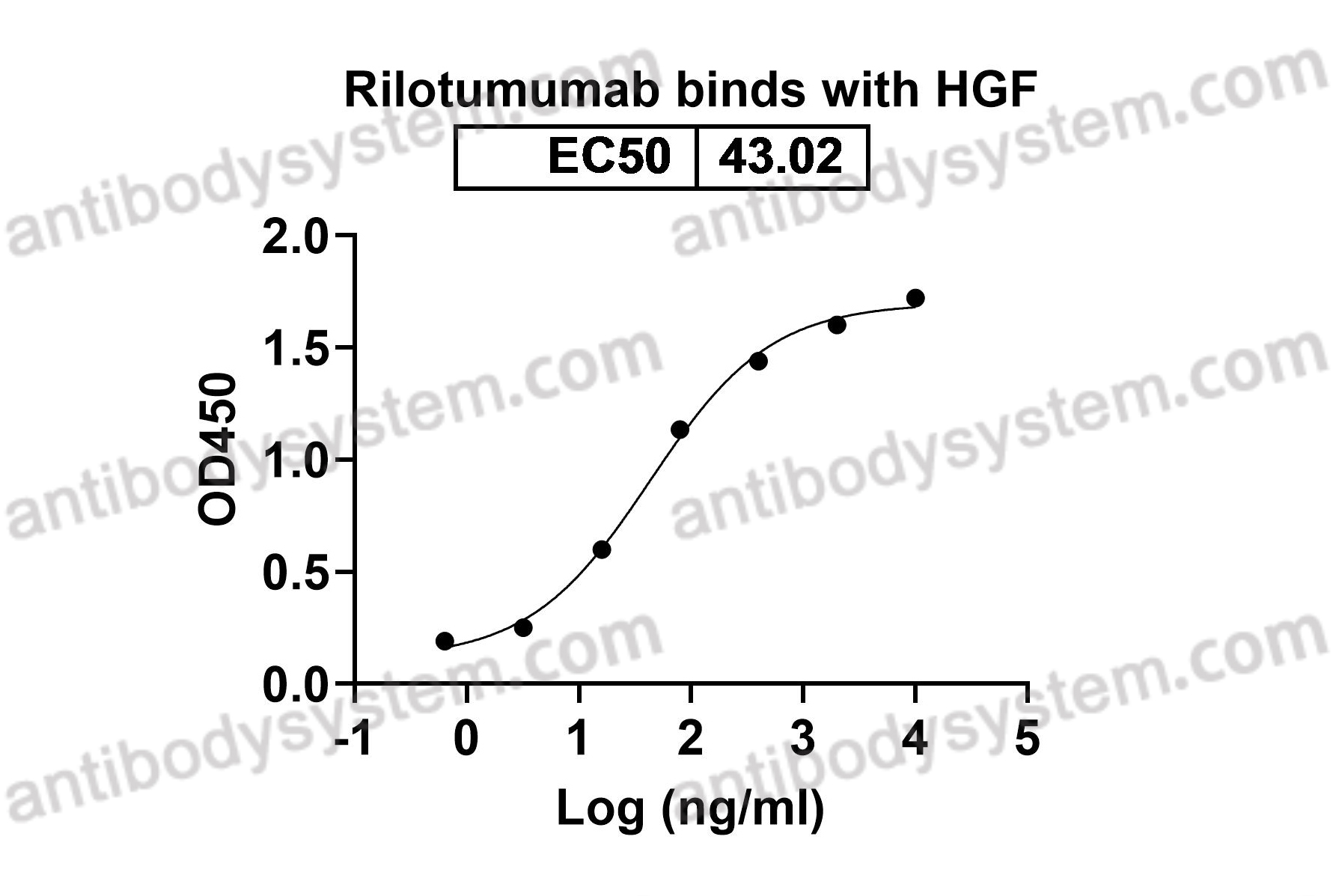
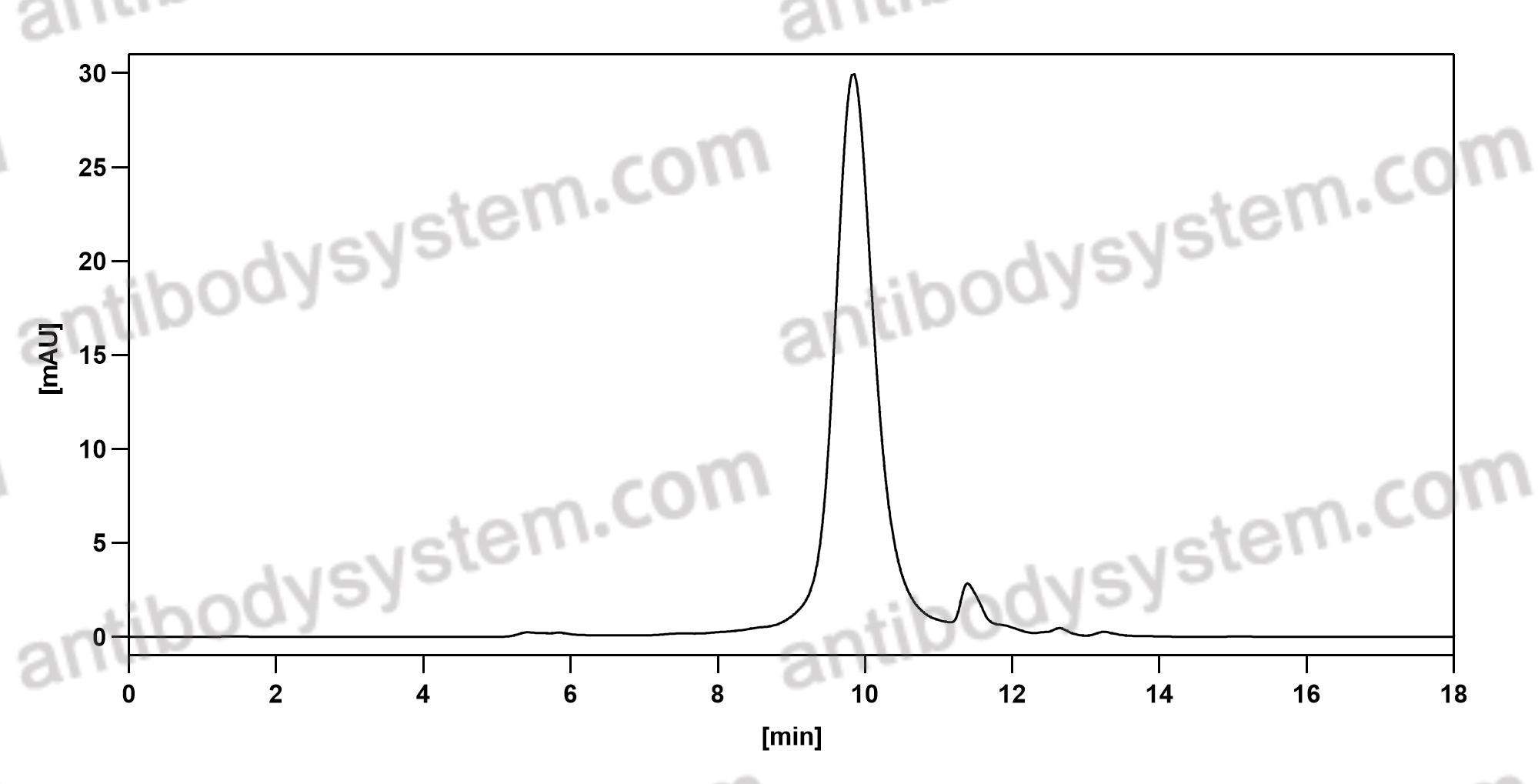
[AMG-102 inhibits proliferation and induces apoptosis of laryngeal squamous cell carcinoma cells by regulating c-Met/PI3K/Akt pathway], PMID: 32135642
[Effect of c-Met inhibitor AMG-102 on radiosensitivity in laryngeal squamous carcinoma cells], PMID: 31874548
89 Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection, PMID: 28280216
A Phase 1/1b tolerability study of rilotumumab alone or in combination with cisplatin and capecitabine in Japanese patients with gastric cancer, PMID: 28973403
A phase II evaluation of AMG 102 (rilotumumab) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: a Gynecologic Oncology Group study, PMID: 24361733
A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer, PMID: 28209746
A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma, PMID: 21297127
A Randomized, Placebo-Controlled, Phase 1b/2 Study of Rilotumumab or Ganitumab in Combination With Platinum-Based Chemotherapy as First-Line Treatment for Extensive-Stage Small-Cell Lung Cancer, PMID: 28601388
Antibodies to watch in 2013: Mid-year update, PMID: 23727858
Assessment of pharmacokinetic interaction between rilotumumab and epirubicin, cisplatin and capecitabine (ECX) in a Phase 3 study in gastric cancer, PMID: 27966237
Biomarker development in MET-targeted therapy, PMID: 27013592
Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol, PMID: 33125909
c-MET as a potential therapeutic target and biomarker in cancer, PMID: 22128285
C-MET inhibitors in the treatment of lung cancer, PMID: 25266653
Combination of HGF/MET-targeting agents and other therapeutic strategies in cancer, PMID: 33497758
Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors, PMID: 24959087
Evaluation of short-term effectiveness of eight targeted agents combined with chemotherapy for treating esophageal-gastric junction adenocarcinoma: A network meta-analysis, PMID: 28708307
Evaluation of the antitumor effects of rilotumumab by PET imaging in a U-87 MG mouse xenograft model, PMID: 23454250
Exposure-response analysis of rilotumumab in gastric cancer: the role of tumour MET expression, PMID: 25584489
FOLFOX alone or combined with rilotumumab or panitumumab as first-line treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): a randomised, open-label, three-arm phase II trial, PMID: 31129386
Gastric Cancer: New Drugs - New Strategies, PMID: 26674336
Gastric cancer--epidemiologic and clinical aspects, PMID: 25167943
GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevent primary tumor growth and metastasis in Ewing sarcoma, PMID: 31621900
Hepatocyte growth factor inhibition: a novel therapeutic approach in pancreatic cancer, PMID: 26766740
How prognostic and predictive biomarkers are transforming our understanding and management of advanced gastric cancer, PMID: 25142842
Incomplete target neutralization by the anti-cancer antibody rilotumumab, PMID: 26750997
Mechanistically detailed systems biology modeling of the HGF/Met pathway in hepatocellular carcinoma, PMID: 31452933
Met, IGF1R, and other new targets in upper GI malignancies, PMID: 23873272
Modulation of c-Met signaling and cellular sensitivity to radiation: potential implications for therapy, PMID: 23423860
Monoclonal antibodies for treating gastric cancer: promises and pitfalls, PMID: 26971395
Pharmacokinetics and pharmacodynamics of rilotumumab: a decade of experience in preclinical and clinical cancer research, PMID: 25912961
Phase 1/2 study of rilotumumab (AMG 102), a hepatocyte growth factor inhibitor, and erlotinib in patients with advanced non-small cell lung cancer, PMID: 28472537
Phase II Study to Evaluate the Efficacy and Safety of Rilotumumab and Bevacizumab in Subjects with Recurrent Malignant Glioma, PMID: 29666296
Population pharmacokinetics of rilotumumab, a fully human monoclonal antibody against hepatocyte growth factor, in cancer patients, PMID: 24186235
Potential role of rilotumumab in the treatment of gastric cancer, PMID: 25524381
Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer, PMID: 24919569
Rilotumumab exposure-response relationship in patients with advanced or metastatic gastric cancer, PMID: 25712685
Rilotumumab extends PFS in gastric cancer, PMID: 25185191
Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study, PMID: 24965569
Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial, PMID: 28958504
Rilotumumab, a mAb against human hepatocyte growth factor for the treatment of cancer, PMID: 19649990
Targeted MET inhibition in castration-resistant prostate cancer: a randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone, PMID: 23136195
Targeted therapies for advanced and metastatic adenocarcinoma of the gastroesophageal junction: is there something new?, PMID: 27568322
Targeting c-Met in triple negative breast cancer: preclinical studies using the c-Met inhibitor, Cpd A, PMID: 32318883
Targeting the C-MET/HGF Signaling Pathway in Pancreatic Ductal Adenocarcinoma, PMID: 30636579
Targeting the HGF/MET pathway in gastric cancer, PMID: 24965570
The efficacy and safety of targeted therapy with or without chemotherapy in advanced gastric cancer treatment: a network meta-analysis of well-designed randomized controlled trials, PMID: 29455269
The emerging role of MET/HGF inhibitors in oncology, PMID: 23453860
The persistent promise of combining HGF/MET and EGFR inhibition in non-small cell lung cancer, PMID: 28472534
The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases, PMID: 19412429
Computational Drug Discovery in Ankylosing Spondylitis-Induced Osteoporosis Based on Data Mining and Bioinformatics Analysis., PMID:36716856
Rilotumumab Resistance Acquired by Intracrine Hepatocyte Growth Factor Signaling., PMID:36672409
Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases., PMID:35458666
Comparative efficacy and safety of anti-HGF/MET pathway agents plus chemotherapy versus chemotherapy alone as first-line treatment in advanced gastric cancer: a protocol for a systematic review and meta-analysis., PMID:34952869
Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients., PMID:34771724
Combination of HGF/MET-targeting agents and other therapeutic strategies in cancer., PMID:33497758
Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol., PMID:33125909
Targeting c-Met in triple negative breast cancer: preclinical studies using the c-Met inhibitor, Cpd A., PMID:32318883
[AMG-102 inhibits proliferation and induces apoptosis of laryngeal squamous cell carcinoma cells by regulating c-Met/PI3K/Akt pathway]., PMID:32135642
[Effect of c-Met inhibitor AMG-102 on radiosensitivity in laryngeal squamous carcinoma cells]., PMID:31874548
GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevent primary tumor growth and metastasis in Ewing sarcoma., PMID:31621900
Mechanistically detailed systems biology modeling of the HGF/Met pathway in hepatocellular carcinoma., PMID:31452933
FOLFOX alone or combined with rilotumumab or panitumumab as first-line treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): a randomised, open-label, three-arm phase II trial., PMID:31129386
Targeting the C-MET/HGF Signaling Pathway in Pancreatic Ductal Adenocarcinoma., PMID:30636579
Phase II Study to Evaluate the Efficacy and Safety of Rilotumumab and Bevacizumab in Subjects with Recurrent Malignant Glioma., PMID:29666296
The efficacy and safety of targeted therapy with or without chemotherapy in advanced gastric cancer treatment: a network meta-analysis of well-designed randomized controlled trials., PMID:29455269
A Phase 1/1b tolerability study of rilotumumab alone or in combination with cisplatin and capecitabine in Japanese patients with gastric cancer., PMID:28973403
Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial., PMID:28958504
Evaluation of short-term effectiveness of eight targeted agents combined with chemotherapy for treating esophageal-gastric junction adenocarcinoma: A network meta-analysis., PMID:28708307
A Randomized, Placebo-Controlled, Phase 1b/2 Study of Rilotumumab or Ganitumab in Combination With Platinum-Based Chemotherapy as First-Line Treatment for Extensive-Stage Small-Cell Lung Cancer., PMID:28601388
Phase 1/2 study of rilotumumab (AMG 102), a hepatocyte growth factor inhibitor, and erlotinib in patients with advanced non-small cell lung cancer., PMID:28472537
The persistent promise of combining HGF/MET and EGFR inhibition in non-small cell lung cancer., PMID:28472534
89Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection., PMID:28280216
A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer., PMID:28209746
Assessment of pharmacokinetic interaction between rilotumumab and epirubicin, cisplatin and capecitabine (ECX) in a Phase 3 study in gastric cancer., PMID:27966237
Targeted therapies for advanced and metastatic adenocarcinoma of the gastroesophageal junction: is there something new?, PMID:27568322
Biomarker development in MET-targeted therapy., PMID:27013592
Monoclonal antibodies for treating gastric cancer: promises and pitfalls., PMID:26971395
Hepatocyte growth factor inhibition: a novel therapeutic approach in pancreatic cancer., PMID:26766740
Incomplete target neutralization by the anti-cancer antibody rilotumumab., PMID:26750997
Pharmacokinetics and pharmacodynamics of rilotumumab: a decade of experience in preclinical and clinical cancer research., PMID:25912961
Rilotumumab exposure-response relationship in patients with advanced or metastatic gastric cancer., PMID:25712685
Exposure-response analysis of rilotumumab in gastric cancer: the role of tumour MET expression., PMID:25584489
Potential role of rilotumumab in the treatment of gastric cancer., PMID:25524381
C-MET inhibitors in the treatment of lung cancer., PMID:25266653
Rilotumumab extends PFS in gastric cancer., PMID:25185191
Gastric cancer--epidemiologic and clinical aspects., PMID:25167943
How prognostic and predictive biomarkers are transforming our understanding and management of advanced gastric cancer., PMID:25142842
Targeting the HGF/MET pathway in gastric cancer., PMID:24965570
Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study., PMID:24965569
Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors., PMID:24959087
Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer., PMID:24919569
Gastric Cancer: New Drugs - New Strategies., PMID:26674336
A phase II evaluation of AMG 102 (rilotumumab) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: a Gynecologic Oncology Group study., PMID:24361733
Population pharmacokinetics of rilotumumab, a fully human monoclonal antibody against hepatocyte growth factor, in cancer patients., PMID:24186235
Met, IGF1R, and other new targets in upper GI malignancies., PMID:23873272
Antibodies to watch in 2013: Mid-year update., PMID:23727858
Evaluation of the antitumor effects of rilotumumab by PET imaging in a U-87 MG mouse xenograft model., PMID:23454250
The emerging role of MET/HGF inhibitors in oncology., PMID:23453860
Modulation of c-Met signaling and cellular sensitivity to radiation: potential implications for therapy., PMID:23423860