Catalog No.
DHD12605
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa-[scFv]2
Clonality
Monoclonal
Target
Vascular endothelial growth factor A, VPF, VEGFA, VEGF, Vascular permeability factor, VEGF-A, Programmed cell death protein 1, Protein PD-1, hPD-1, PD1, PDCD1, CD279
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
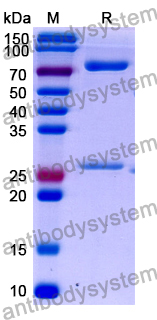
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P15692 & Q15116
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
PBS, pH 6.0, 0.2M Arg
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Tetravalent Bispecific, AK 112, AK-112, AK112 CAS: 2428381-53-5
Clone ID
Ivonescimab
Bispecific Antibodies in Non-Small Cell Lung Cancer: From Targeted Innovation to Real-World Integration., PMID:40397846
Efficacy and safety of distinct regimens for individuals with advanced EGFR-mutated non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitors: a systematic review and network meta-analysis., PMID:40396122
The evolving immuno-angiogenic paradigm in NSCLC: lessons from ivonescimab., PMID:40329050
Unveiling the Synergistic Potential: Bispecific Antibodies in Conjunction with Chemotherapy for Advanced Non-Small-Cell Lung Cancer Treatment., PMID:40277763
Ivonescimab in non-small cell lung cancer: harmonizing immunotherapy and anti-angiogenesis., PMID:40162997
Safety, Pharmacokinetics, and Pharmacodynamics Evaluation of Ivonescimab, a Novel Bispecific Antibody Targeting PD-1 and VEGF, in Chinese Patients With Advanced Solid Tumors., PMID:40114411
Ivonescimab versus pembrolizumab for PD-L1-positive non-small cell lung cancer (HARMONi-2): a randomised, double-blind, phase 3 study in China., PMID:40057343
Ivonescimab in advanced NSCLC: is progression-free survival enough, or are overall survival data also needed?, PMID:40057331
Design of a fragment crystallizable-engineered tetravalent bispecific antibody targeting programmed cell death-1 and vascular endothelial growth factor with cooperative biological effects., PMID:40034861
Efficacy and Safety of Ivonescimab in the Treatment of Advanced Non-small Cell Lung Cancer (NSCLC): A Systematic Review., PMID:39944427
Finding the right HARMONi-A., PMID:39830738
Antibodies to watch in 2025., PMID:39711140
Ivonescimab Plus Chemotherapy in Patients With EGFR Variant Non-Small Cell Lung Cancer-Reply., PMID:39661384
Ivonescimab Plus Chemotherapy in Patients With EGFR Variant Non-Small Cell Lung Cancer., PMID:39661367
Ivonescimab Plus Chemotherapy in Patients With EGFR Variant Non-Small Cell Lung Cancer., PMID:39661345
Brief Report: Ivonescimab Combined With Etoposide Plus Carboplatin as First-Line Treatment for Extensive-Stage SCLC: Results of a Phase 1b Clinical Trial., PMID:39490738
EGFR-mutated NSCLC: A roadmap to treatment sequences., PMID:39276767
Ivonescimab: First Approval., PMID:39073550
Ivonescimab Plus Chemotherapy in Non-Small Cell Lung Cancer With EGFR Variant: A Randomized Clinical Trial., PMID:38820549
Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors., PMID:38642937
Antibodies to watch in 2024., PMID:38178784
A Phase 1b Study of Ivonescimab, a Programmed Cell Death Protein-1 and Vascular Endothelial Growth Factor Bispecific Antibody, as First- or Second-Line Therapy for Advanced or Metastatic Immunotherapy-Naive NSCLC., PMID:37879536