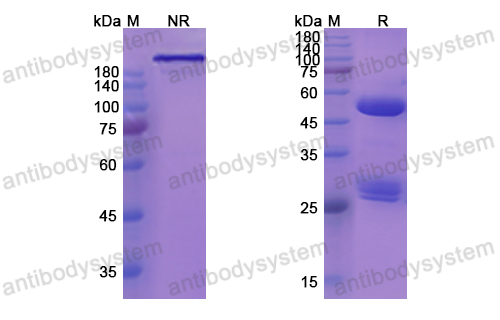
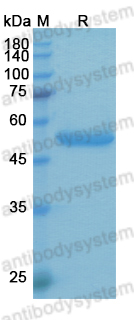
MOR202, a novel anti-CD38 monoclonal antibody, in patients with relapsed or refractory multiple myeloma: a first-in-human, multicentre, phase 1-2a trial, PMID: 32171061
Emerging agents and regimens for multiple myeloma, PMID: 33168044
CD38 antibodies in multiple myeloma: back to the future, PMID: 29118010
Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond, PMID: 29257139
Monoclonal antibodies targeting CD38 in hematological malignancies and beyond, PMID: 26864107
Resistance Mechanisms Towards CD38-Directed Antibody Therapy in Multiple Myeloma, PMID: 32331242
Immunomodulatory effects of CD38-targeting antibodies, PMID: 29702148
CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance, PMID: 30294326
Lenalidomide enhances MOR202-dependent macrophage-mediated effector functions via the vitamin D pathway, PMID: 29654274
Combination of lenalidomide and vitamin D enhances MOR202-mediated cytotoxicity of macrophages: It takes three to tango, PMID: 30713598
Sequential CD38 monoclonal antibody retreatment leads to deep remission in a patient with relapsed/refractory multiple myeloma, PMID: 33353443
Reprint of "Immunomodulatory effects of CD38-targeting antibodies", PMID: 30826127
CD38: targeted therapy in multiple myeloma and therapeutic potential for solid cancers, PMID: 32822558
CD38-targeting antibodies in multiple myeloma: mechanisms of action and clinical experience, PMID: 29465271
Clinical and Pharmacologic Features of Monoclonal Antibodies and Checkpoint Blockade Therapy in Multiple Myeloma, PMID: 29756564
CD38 and Anti-CD38 Monoclonal Antibodies in AL Amyloidosis: Targeting Plasma Cells and beyond, PMID: 32531894
A Reduction in B, T, and Natural Killer Cells Expressing CD38 by TAK-079 Inhibits the Induction and Progression of Collagen-Induced Arthritis in Cynomolgus Monkeys, PMID: 31085699
CD38 as Immunotherapeutic Target in Light Chain Amyloidosis and Multiple Myeloma-Association With Molecular Entities, Risk, Survival, and Mechanisms of Upfront Resistance, PMID: 30079070
Daratumumab (anti-CD38)- and elotuzumab (anti-SLAMF7)-based treatments for refractory POEMS syndrome: a single-center case series., PMID:40528367
Retreatment With Anti-CD38-Based Combinations in Multiple Myeloma in Real-Life: Results From the Emmy Cohort Study., PMID:40518372
PSGL-1 is a phagocytosis checkpoint that enables tumor escape from macrophage clearance., PMID:40512837
Efficacy and safety of anti-CD38 monoclonal antibodies in patients with newly diagnosed multiple myeloma: an updated systematic review and meta-analysis based on randomized controlled trials., PMID:40501427
Daratumumab for Treatment of Acute Antibody-Mediated Rejection in a Pediatric Kidney Transplant Recipient., PMID:40490910
Antibody-mediated ratiometric delivery of FLT3 and CDK4/6 dual inhibitors for targeted treatment of acute myeloid leukemia., PMID:40482924
Comparative Efficacy and Safety of Daratumumab-Integrated Quadruple Therapy Versus Triple Therapy in Transplant-Eligible Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-Analysis., PMID:40473556
Peripheral memory B cell population maintenance and long-term survival after perioperative chemoimmunotherapy in NSCLC (NADIM trial)., PMID:40468805
FDA Approval Summary: Idecabtagene Vicleucel for the Treatment of Triple-Class Exposed, Relapsed or Refractory Multiple Myeloma., PMID:40465403
Functional multiomics reveals genetic and pharmacologic regulation of surface CD38 in multiple myeloma., PMID:40453054
A ROS-Responsive Dual-Targeting Drug Nanocarrier Serving as a GSI Synergist and Ferroptosis Sensitizer for T-Cell Acute Lymphoblastic Leukemia., PMID:40448620
Population Pharmacokinetics for Belantamab Mafodotin Monotherapy and Combination Therapies in Patients with Relapsed/Refractory Multiple Myeloma., PMID:40447948
Targeting CD38 in Antibody-Mediated Rejection., PMID:40444214
Real-World Treatment Patterns and Survival Outcomes of Patients with Relapsed/Refractory Multiple Myeloma Treated with a Selinexor-Containing Triplet-Based Regimen., PMID:40422527
Reduced immune response to SARS-CoV-2 infection in the elderly after 6 months., PMID:40416973
Daratumumab monotherapy as a desensitization strategy prior to cardiac transplantation., PMID:40407767
Practice-changing updates on multiple myeloma: highlights from the 2024 ASH annual meeting., PMID:40361170
Phase 1 study of talquetamab, a humanized GPRC5D x CD3 bispecific antibody, in Japanese patients with relapsed/refractory MM., PMID:40343678
Real-world analysis of treatment patterns, effectiveness, and safety of daratumumab-based regimens in Chinese patients with newly diagnosed or relapsed/refractory multiple myeloma., PMID:40335949
CD38-targeted attenuated interferon alpha immunocytokine activates both innate and adaptive immune cells to drive anti-tumor activity., PMID:40315226
Effect of felzartamab on the molecular phenotype of antibody-mediated rejection in kidney transplant biopsies., PMID:40301559
Kinetics of the humoral and cellular immune response up to 1 year from mpox virus infection., PMID:40294869
Peripheral T-cell subset activation in NMDAR encephalitis: Insights into pathogenesis and biomarker potential for disease monitoring., PMID:40288549
Real-World Data on the Efficacy of Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma and Amplification 1q., PMID:40282438
Consensus Guidelines and Recommendations for The CD38 Monoclonal Antibody-based Quadruplet Therapy and Management in Clinical Practice for Newly Diagnosed Multiple Myeloma: From the Pan-Pacific Multiple Myeloma Working Group., PMID:40271095
Multiple myeloma with 1q gain/amplification exhibits reduced CD38 expression via interleukin-6 receptor overexpression., PMID:40259818
Targeting CD38 immunometabolic checkpoint improves metabolic fitness and cognition in a mouse model of Alzheimer's disease., PMID:40254603
BCMA CAR T cells in a patient with relapsing idiopathic inflammatory myositis after initial and repeat therapy with CD19 CAR T cells., PMID:40245922
The de novo DNA methyltransferase 3B is a novel epigenetic regulator of MYC in multiple myeloma, representing a promising therapeutic target to counter relapse., PMID:40241199
Treatment refractoriness and response rates in patients with relapsed/refractory multiple myeloma: a retrospective analysis of real-world data., PMID:40199119
Efficacy and Safety of Quadruplet Therapy in Newly Diagnosed Transplant-Eligible Multiple Myeloma: A Systematic Review and Meta-Analysis., PMID:40176408
Impact of Protein Kinase C Activation and Monoclonal Antibodies on Immune Checkpoint Regulation and B Cell Function in Patients with Chronic Lymphocytic Leukemia., PMID:40149717
A systematic review and meta-analysis of phase III randomized controlled trials to assess the risk of pneumonia, URTIs, and VTE in multiple myeloma patients treated with isatuximab., PMID:40124649
Outcomes of Newly Diagnosed Multiple Myeloma Patients Requiring Dialysis., PMID:40122729
The effects of intragraft CD38+ B cell on chronic active antibody mediated rejection in kidney transplantation., PMID:40111715
T-cell activation and senescence in asymptomatic HIV/Leishmania infantum co-infection., PMID:40096173
Real-World Outcomes of Newly Diagnosed Multiple Myeloma Patients Treated Before the Era of Anti-CD38 Antibodies: The EMMY Cohort From 2017 to 2020., PMID:40087879
Early daratumumab therapy improves renal outcomes in newly diagnosed myeloma patients admitted with kidney injury., PMID:40085948
How First-Line Therapy is Changing in non-Transplant Eligible Multiple Myeloma Patients., PMID:40084107
Novelties on Multiple Myeloma from the Main 2024 Hematology Conferences., PMID:40084104
How First-Line Therapy is Changing in Transplant-Eligible Multiple Myeloma Patients., PMID:40084095
Evolution of Survival in Patients with Multiple Myeloma over Two Decades: A Real-World Experience from a Medium-Level Hospital., PMID:40075641
Efficacy of Combined CD38 and PD1 Inhibition with Isatuximab and Cemiplimab for Relapsed/Refractory NK/T-Cell Lymphoma., PMID:40073374
Gene-modified NK cells expressing CD64 and preloaded with HIV-specific BNAbs target autologous HIV-1-infected CD4+ T cells by ADCC., PMID:40073240
CD38 in the pathobiology of cutaneous T-cell lymphoma and the potential for combination therapeutic intervention., PMID:40057636
CD31 + naïve T cells associate with immunosenescence and responsiveness to multiple vaccines in older adults., PMID:40055790
Treatment of Multiple Myeloma in Patients Refractory to Daratumumab/Anti-CD38 Monoclonal Antibodies: A Systematic Review., PMID:40052837
Outcomes of triple-class refractory multiple myeloma patients at a single clinic: A retrospective study., PMID:40037111
Monoclonal anti-CD38 therapy in human myeloma: retrospects and prospects., PMID:40013150