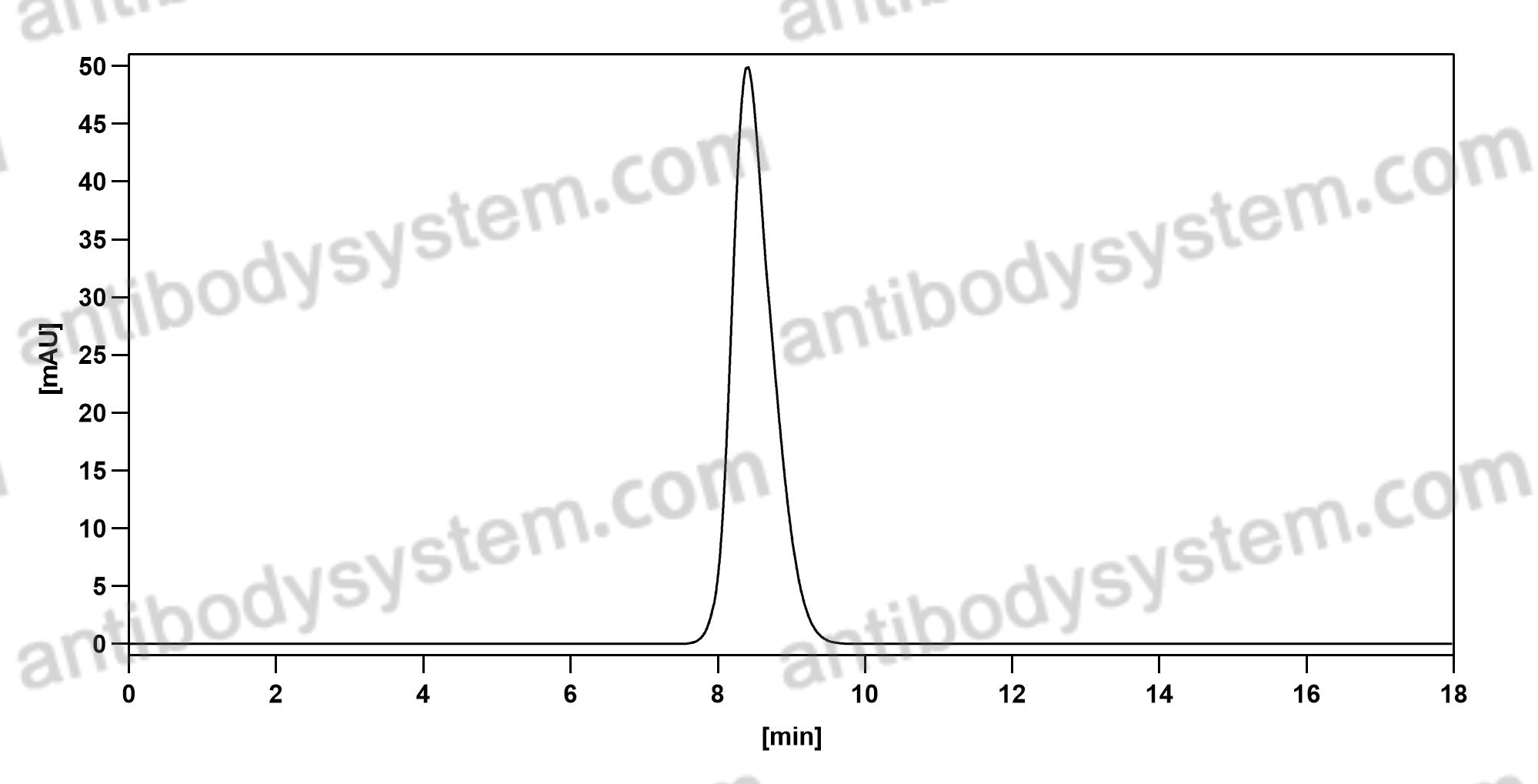
Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: an international cohort study, PMID: 29437868
Agalsidase Alfa, PMID: 30601613
Clinical observation of patients with Fabry disease after switching from agalsidase beta (Fabrazyme) to agalsidase alfa (Replagal), PMID: 22878505
Clinical observation of patients with Fabry disease after switching from agalsidase beta (Fabrazyme) to agalsidase alfa (Replagal), PMID: 22498845
Fabry Disease Therapy: State-of-the-Art and Current Challenges, PMID: 33379210
Successful management of enzyme replacement therapy in related fabry disease patients with severe adverse events by switching from agalsidase Beta (fabrazyme(®)) to agalsidase alfa (replagal (®)), PMID: 24718841
Enzyme replacement therapy for Anderson-Fabry disease, PMID: 27454104
Genetics and Gene Therapy of Anderson-Fabry Disease, PMID: 29618309
Agalsidase alfa: a review of its use in the management of Fabry disease, PMID: 22946754
Effects of switching from agalsidase Beta to agalsidase alfa in 10 patients with anderson-fabry disease, PMID: 23430546
Agalsidase alfa for the treatment of Fabry disease: new data on clinical efficacy and safety, PMID: 19236256
Altered immune phenotypes in subjects with Fabry disease and responses to switching from agalsidase alfa to agalsidase beta, PMID: 30972193
Fabry disease during the COVID-19 pandemic. Why and how treatment should be continued, PMID: 32561366
Agalsidase alfa--a preparation for enzyme replacement therapy in Anderson-Fabry disease, PMID: 12036428
Switch to agalsidase alfa after shortage of agalsidase beta in Fabry disease: a systematic review and meta-analysis of the literature, PMID: 27608175
The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts, PMID: 30775256
Treatment switch in Fabry disease- a matter of dose?, PMID: 32522756
Switch from agalsidase beta to agalsidase alfa in the enzyme replacement therapy of patients with Fabry disease in Latin America, PMID: 28643672
Clinical observations on enzyme replacement therapy in patients with Fabry disease and the switch from agalsidase beta to agalsidase alfa, PMID: 24388678
Agalsidase Beta, PMID: 29999661
Reduction of Plasma Globotriaosylsphingosine Levels After Switching from Agalsidase Alfa to Agalsidase Beta as Enzyme Replacement Therapy for Fabry Disease, PMID: 26303609
[The switch of enzyme therapy in Fabry disease], PMID: 25098458
Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg, PMID: 17622343
Agalsidase alfa and agalsidase beta in the treatment of Fabry disease: does the dose really matter?, PMID: 25010055
Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice, PMID: 16372133
Enzyme replacement therapy for Anderson-Fabry disease, PMID: 23450571
Effect and Tolerability of Agalsidase Alfa in Patients with Fabry Disease Who Were Treatment Naïve or Formerly Treated with Agalsidase Beta or Agalsidase Alfa, PMID: 25822820
Update on role of agalsidase alfa in management of Fabry disease, PMID: 21552486
Efficacy and safety of enzyme-replacement-therapy with agalsidase alfa in 36 treatment-naïve Fabry disease patients, PMID: 28592315
Enzyme replacement therapy for Anderson-Fabry disease: A complementary overview of a Cochrane publication through a linear regression and a pooled analysis of proportions from cohort studies, PMID: 28296917
Home infusion program for Fabry disease: experience with agalsidase alfa in Argentina, PMID: 23335703
Anderson-Fabry disease in children, PMID: 23448455
Effect of reduced agalsidase Beta dosage in fabry patients: the Australian experience, PMID: 23430871
Fabry disease, enzyme replacement therapy and the significance of antibody responses, PMID: 22037707
Fabry disease under enzyme replacement therapy-new insights in efficacy of different dosages, PMID: 29186537
Enzyme replacement therapy for Anderson-Fabry disease, PMID: 20464743
Agalsidase alfa (Replagal) in the treatment of Anderson-Fabry disease, PMID: 19707338
Efficacy of enzyme replacement therapy in Fabry disease, PMID: 15320778
Enzyme replacement therapy for Fabry disease: lessons from two alpha-galactosidase A orphan products and one FDA approval, PMID: 15268683
Renal complications of Fabry disease, PMID: 23448456
Recommendations on reintroduction of agalsidase Beta for patients with fabry disease in europe, following a period of shortage, PMID: 23430520
Enzyme replacement therapy of Fabry disease, PMID: 16077182
Fabry nephropathy: a review - how can we optimize the management of Fabry nephropathy?, PMID: 24886109
Enzyme replacement therapy in Fabry disease: comparison of agalsidase alfa and agalsidase beta, PMID: 18701330
Enzyme replacement therapy in patients with Fabry disease: state of the art and review of the literature, PMID: 22963910
Anti-drug antibody formation in Japanese Fabry patients following enzyme replacement therapy, PMID: 33072516
Oral Migalastat HCl Leads to Greater Systemic Exposure and Tissue Levels of Active α-Galactosidase A in Fabry Patients when Co-Administered with Infused Agalsidase, PMID: 26252393
The pharmacology of multiple regimens of agalsidase alfa enzyme replacement therapy for Fabry disease, PMID: 17700388
Enzyme replacement therapy in Fabry disease: influence on cardiac manifestations, PMID: 20345350
Gateways to clinical trials, PMID: 15672123
Current status of the immunogenicity of enzyme replacement therapy in fabry disease., PMID:40420145
Progress and Challenges in the Treatment of Fabry Disease., PMID:40310476
Impact of enzyme replacement therapy and migalastat on disease progression in females with fabry disease., PMID:39980015
Efforts of the Pharmaceuticals and Medical Devices Agency of Japanese regulatory agency in supporting biosimilar development and disseminate information., PMID:39951116
Effects of Current Therapies on Disease Progression in Fabry Disease: A Narrative Review for Better Patient Management in Clinical Practice., PMID:39636569
A phase III, open-label clinical trial evaluating pegunigalsidase alfa administered every 4 weeks in adults with Fabry disease previously treated with other enzyme replacement therapies., PMID:39381863
Establishing Treatment Effectiveness in Fabry Disease: Observation-Based Recommendations for Improvement., PMID:39273698
Comparative study on incorporation of three recombinant human α-galactosidase A drugs (agalsidases) into cultured fibroblasts and organs/tissues of Fabry mice., PMID:39257531
[What is confirmed in the treatment of Fabry's disease?]., PMID:39105759
A systematic literature review on the health-related quality of life and economic burden of Fabry disease., PMID:38689282
Pegunigalsidase alfa: a novel, pegylated recombinant alpha-galactosidase enzyme for the treatment of Fabry disease., PMID:38680424
Response to commentary: Head-to-head trial of pegunigalsidase alfa versus agalsidase beta in patients with Fabry disease and deteriorating renal function: results from the 2-year randomised phase III BALANCE study - determination of immunogenicity., PMID:38589225
Comment to: Head-to-head trial of pegunigalsidase alfa versus agalsidase beta in patients with Fabry disease and deteriorating renal function: results from the 2-year randomised phase III BALANCE study-determination of immunogenicity., PMID:38538083
Successful treatment using agalsidase alfa of a patient with Fabry disease who had anaphylaxis after agalsidase beta: A case report., PMID:38357316
Reducing agalsidase beta infusion time in Fabry patients: low incidence of antibody formation and infusion-associated reactions in an Italian multicenter study., PMID:38308295
Effects of switching from agalsidase-α to agalsidase-β on biomarkers, renal and cardiac parameters, and disease severity in fabry disease forming neutralizing antidrug antibodies: a case report., PMID:38135868
Impact of enzyme replacement therapy and migalastat on left atrial strain and cardiomyopathy in patients with Fabry disease., PMID:38028489
Head-to-head trial of pegunigalsidase alfa versus agalsidase beta in patients with Fabry disease and deteriorating renal function: results from the 2-year randomised phase III BALANCE study., PMID:37940383
Clinical study of left ventricular structure and function in patients with Anderson-Fabry disease before and after enzyme replacement therapy., PMID:37883130
Characterization of pre-existing anti-PEG and anti-AGAL antibodies towards PRX-102 in patients with Fabry disease., PMID:37818380
New insights in efficacy of different ERT dosages in Fabry disease: Switch and switch-back studies data following agalsidase beta shortage. Update of systematic review., PMID:39669233
Assessment and impact of dose escalation on anti-drug antibodies in Fabry disease., PMID:36569886
New insights in efficacy of different enzyme replacement therapy dosages in Fabry disease: Switch studies data following agalsidase beta shortage., PMID:36373246
Monitoring of anti-drug antibodies and disease-specific biomarkers in three patients from a Japanese Fabry family treated with enzyme replacement therapy., PMID:36205882
A Fabry Disease Patient Who Developed Hypersensitivity Reaction against Agalsidase Beta following COVID-19 Infection., PMID:36174537
Pre-existing anti-drug antibodies in Fabry disease show less affinity for pegunigalsidase alfa., PMID:35990747
Fabry Disease: Current and Novel Therapeutic Strategies. A Narrative Review., PMID:35652398
Mechanisms of Neutralizing Anti-drug Antibody Formation and Clinical Relevance on Therapeutic Efficacy of Enzyme Replacement Therapies in Fabry Disease., PMID:34748189
[The treatment for Fabry disease: focus on agalsidase alpha and beta]., PMID:34647542
Migalastat Tissue Distribution: Extrapolation From Mice to Humans Using Pharmacokinetic Modeling and Comparison With Agalsidase Beta Tissue Distribution in Mice., PMID:33876577
Fabry Disease: The Current Treatment Landscape., PMID:33721270
Fabry Disease Therapy: State-of-the-Art and Current Challenges., PMID:33379210
Anti-drug antibody formation in Japanese Fabry patients following enzyme replacement therapy., PMID:33072516
Globotriaosylsphingosine (lyso-Gb3) and analogues in plasma and urine of patients with Fabry disease and correlations with long-term treatment and genotypes in a nationwide female Danish cohort., PMID:32963035
Surface plasmon resonance analysis of complex formation of therapeutic recombinant lysosomal enzymes with domain 9 of human cation-independent mannose 6-phosphate receptor., PMID:32884906
Predicting the Development of Anti-Drug Antibodies against Recombinant alpha-Galactosidase A in Male Patients with Classical Fabry Disease., PMID:32806627
Switch from enzyme replacement therapy to oral chaperone migalastat for treating fabry disease: real-life data., PMID:32647377
Angiokeratomas and treatment with enzyme replacement therapy in a patient with Fabry disease., PMID:32566958
Fabry disease during the COVID-19 pandemic. Why and how treatment should be continued., PMID:32561366
Treatment switch in Fabry disease- a matter of dose?, PMID:32522756
The Changing Landscape of Fabry Disease., PMID:32132142
Efficacy and safety of migalastat in a Japanese population: a subgroup analysis of the ATTRACT study., PMID:31889231
Myocardial Storage, Inflammation, and Cardiac Phenotype in Fabry Disease After One Year of Enzyme Replacement Therapy., PMID:31826677
Response to Gurevich and colleagues: The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: a systematic literature review by a European panel of experts., PMID:31467848
[The Fabry nephropathy: new insight in diagnosis, monitoring and treatment]., PMID:31373466
Why systematic literature reviews in Fabry disease should include all published evidence., PMID:31195166
[Current status and future prospect of enzyme replacement therapy for Fabry disease]., PMID:31142708
Altered immune phenotypes in subjects with Fabry disease and responses to switching from agalsidase alfa to agalsidase beta., PMID:30972193