Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial, PMID: 31189511
Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial, PMID: 29397376
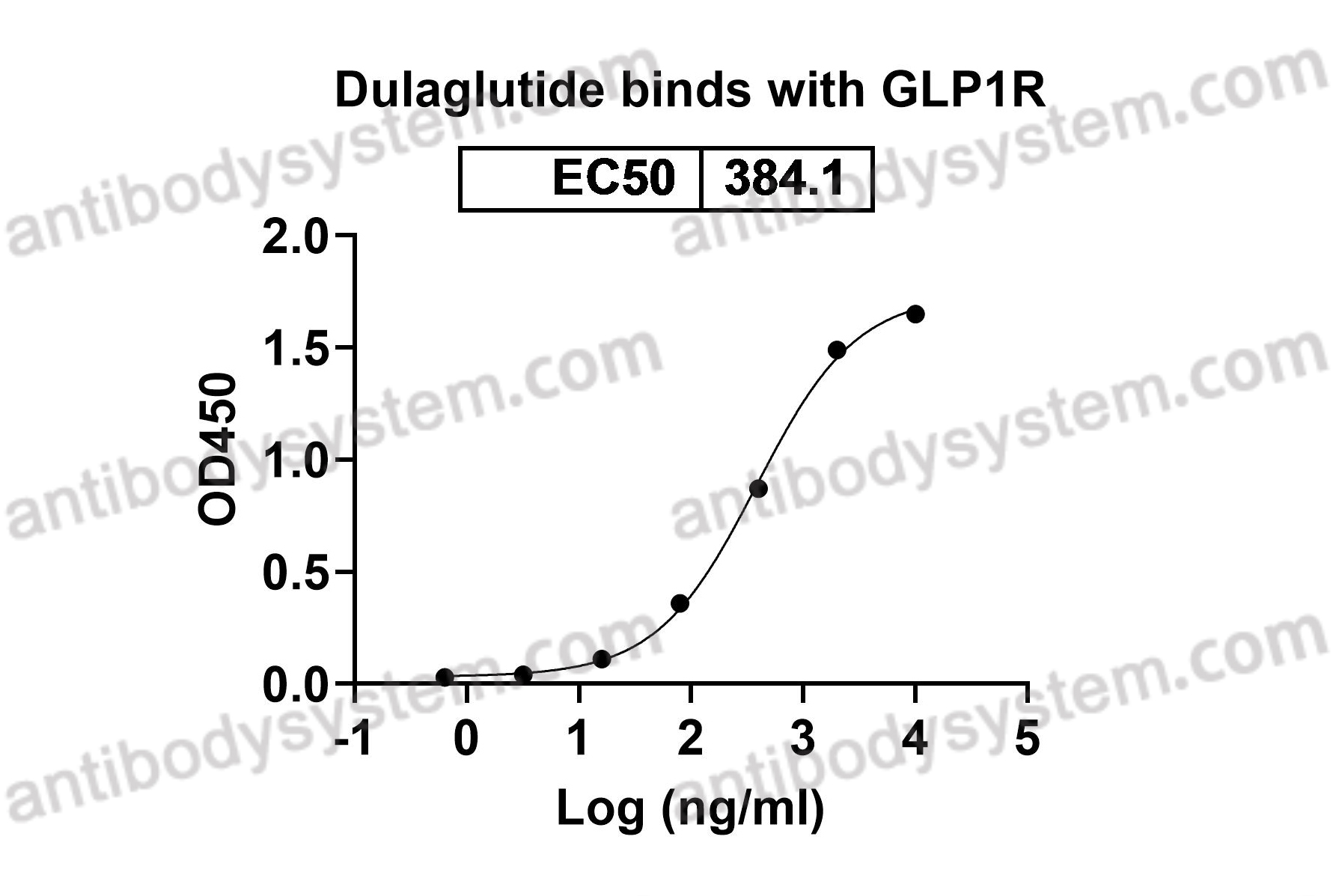
Dulaglutide for the treatment of type 2 diabetes, PMID: 28274140
Dulaglutide: A Review in Type 2 Diabetes, PMID: 32002850
Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial, PMID: 32333876
Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial, PMID: 32562683
Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial, PMID: 31189509
Efficacy and Safety of Dulaglutide 3.0 mg and 4.5 mg Versus Dulaglutide 1.5 mg in Metformin-Treated Patients With Type 2 Diabetes in a Randomized Controlled Trial (AWARD-11), PMID: 33397768
Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial, PMID: 29910024
Dulaglutide, PMID: 31643312
Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial), PMID: 32865597
Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial, PMID: 29483060
Dulaglutide for type 2 diabetes, PMID: 30410215
Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial, PMID: 25018121
Real-World Effectiveness of Dulaglutide in Patients with Type 2 Diabetes Mellitus: A Literature Review, PMID: 32524494
Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: A 52-week open-label, randomized phase III trial, PMID: 30129089
Dulaglutide, PMID: 30000038
The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial, PMID: 31924562
Efficacy and safety of an expanded dulaglutide dose range: A phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin, PMID: 31050143
Effectiveness of dulaglutide vs liraglutide and exenatide once-weekly. A real-world study and meta-analysis of observational studies, PMID: 32109448
Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States, PMID: 30520248
Dulaglutide treatment results in effective glycaemic control in latent autoimmune diabetes in adults (LADA): A post-hoc analysis of the AWARD-2, -4 and -5 Trials, PMID: 29377522
Dulaglutide efficacy, PMID: 31048937
Adherence and persistence among patients with type 2 diabetes initiating dulaglutide compared with semaglutide and exenatide BCise: 6-month follow-up from US real-world data, PMID: 32945083
Assessing patient PREFERence between the dulaglutide pen and the semaglutide pen: A crossover study (PREFER), PMID: 31646727
Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study, PMID: 25912221
Efficacy and safety of dulaglutide in patients with type 2 diabetes: a meta-analysis and systematic review, PMID: 26742577
Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3), PMID: 24842985
Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients With Type 2 Diabetes on Metformin and Glimepiride (AWARD-2), PMID: 26089386
Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1), PMID: 24879836
Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program, PMID: 27102969
Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study, PMID: 26009229
Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5), PMID: 24742660
Dulaglutide: A Review in Type 2 Diabetes, PMID: 26423061
Real-World Effectiveness Analysis of Switching From Liraglutide or Dulaglutide to Semaglutide in Patients With Type 2 Diabetes Mellitus: The Retrospective REALISE-DM Study, PMID: 33367981
Dulaglutide exerts beneficial anti atherosclerotic effects in ApoE knockout mice with diabetes: the earlier, the better, PMID: 33446799
Similar cardiovascular outcomes in patients with diabetes and established or high risk for coronary vascular disease treated with dulaglutide with and without baseline metformin, PMID: 33197271
Dulaglutide mitigates inflammatory response in fibroblast-like synoviocytes, PMID: 31185450
Dulaglutide Shows Sustained Reduction in Glycosylated Hemoglobin Values: 2-Year US Real-world Study Results, PMID: 33256915
Dulaglutide: first global approval, PMID: 25367716
Dulaglutide: an evidence-based review of its potential in the treatment of type 2 diabetes, PMID: 25657615
Dulaglutide and Insulin: How Can the AWARD Studies Help Guide Clinical Practice?, PMID: 32564337
Dulaglutide (Addendum to Commission A15-07) [Internet], PMID: 29144655
Dulaglutide for the treatment of type 2 diabetes, PMID: 24918645
Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials, PMID: 31422062
Dulaglutide: the newest GLP-1 receptor agonist for the management of type 2 diabetes, PMID: 25565404
Dulaglutide slows kidney disease in type 2 diabetes, PMID: 32087784
A novel, long-acting glucagon-like peptide receptor-agonist: dulaglutide, PMID: 26316788
Economic Evaluation of Dulaglutide vs Traditional Therapies: Implications of the Outcomes of the Rewind Study, PMID: 32308446
Mode of administration of dulaglutide: implications for treatment adherence, PMID: 27330280
Comparison of Semaglutide or Dulaglutide Versus Empagliflozin for Risk for Death and Cardiovascular Outcomes Among Patients With Type 2 Diabetes : Two Target Trial Emulation Studies., PMID:40523289
Summary for Patients: Semaglutide or Dulaglutide Versus Empagliflozin for Risk for Death and Cardiovascular Outcomes Among Patients With Type 2 Diabetes., PMID:40523285
Psychiatric adverse events linked to glucagon-like peptide 1 analogues: a disproportionality analysis in American, Canadian and Australian adverse event databases., PMID:40522403
Efficacy of GLP-1-based Therapies on Metabolic Dysfunction-Associated Steatotic Liver Disease and Metabolic Dysfunction-Associated Steatohepatitis: A Systematic Review and Meta-Analysis., PMID:40489581
Effects of Tirzepatide on Patients With Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Cohort Study., PMID:40486301
Treatment with orforglipron, an oral glucagon like peptide-1 receptor agonist, is associated with improvements of CV risk biomarkers in participants with type 2 diabetes or obesity without diabetes., PMID:40481478
Mazdutide, a dual agonist targeting GLP-1R and GCGR, mitigates diabetes-associated cognitive dysfunction: mechanistic insights from multi-omics analysis., PMID:40479843
Cardiovascular Outcomes of Semaglutide vs Dulaglutide in Nonobese Type II Diabetes Patients With HFpEF., PMID:40466245
Real-world evaluation of the efficacy and safety of switching from weekly dulaglutide to weekly semaglutide: The SEMA-SWITCH study., PMID:40450456
Impact of the injectable weight-loss medications, glucagon-like peptide-1 receptor agonists, on reproductive health in non-polycystic ovary syndrome state., PMID:40459435
An overview of recent developments in clinical trials of anti-diabetic drugs., PMID:40457780
Quantifying Critical Quality Attributes of Protein Therapeutics by Sodium Dodecyl Sulfate-Capillary Gel Electrophoresis With Native Fluorescence Detection., PMID:40448278
Comparative effectiveness of semaglutide versus liraglutide, dulaglutide or tirzepatide: a systematic review and meta-analysis., PMID:40444045
Glucagon-Like Peptide-1 Use and Healthcare Resource Utilization for Depression and Anxiety Among Adults with Type 2 Diabetes: 2019 to 2023., PMID:40439835
GLP-1 receptor agonists and the risk for cancer: A meta-analysis of randomized controlled trials., PMID:40437949
Antidiabetic GLP-1 Receptor Agonists Have Neuroprotective Properties in Experimental Animal Models of Alzheimer's Disease., PMID:40430434
Dulaglutide 1.5 mg Significantly Improves Glycemic Control and Lowers LDL-Cholesterol and Body Weight in Romanian Patients with Type 2 Diabetes., PMID:40429530
Pharmacovigilance analysis of neurological adverse events associated with GLP-1 receptor agonists based on the FDA Adverse Event Reporting System., PMID:40413246
Efficacy, Safety, and Future of GLP-1 Receptor Agonists: A Systematic Literature Review and Meta-Analysis., PMID:40409279
Heterogeneity in response to GLP-1 receptor agonists in type 2 diabetes in real-world clinical practice: insights from the DPV register - an IMI-SOPHIA study., PMID:40404820
Enhancing GLP-1 expression via IVT mRNA and fusion protein technology for diabetes therapy., PMID:40393145
Association of Glucagon-Like Peptide-1 Receptor Agonists With Optic Nerve and Retinal Adverse Events: A Population-Based Observational Study Across 180 Countries., PMID:40383360
The role of dulaglutide in the treatment of alcohol use disorder: a case report., PMID:40375882
Survival Benefits of GLP-1 Receptor Agonists in Patients with Neuroendocrine Neoplasms: A Large-Scale Propensity-Matched Cohort Study., PMID:40361517
Glucagon-Like Peptide-1 Receptor Agonists Use Does Not Increase the Risk for Acute Pancreatitis and Is Associated with Lower Complications in Patients with Type 2 Diabetes Who Develop Acute Pancreatitis: A Multi-Center Analysis., PMID:40358430
Euglycemic Diabetic Ketoacidosis in the Setting of Dulaglutide Use., PMID:40357083
Summary for Patients: Comparison of Dose Escalation Versus Switching to Tirzepatide Among People With Type 2 Diabetes Inadequately Controlled on Lower Doses of Dulaglutide., PMID:40340500
A Descriptive Analysis from VigiAccess on Drug-related Problems Associated with the Glucagon-like Peptide-1 Receptor Agonists., PMID:40337971
A Comprehensive Review on the Pharmacokinetics and Drug-Drug Interactions of Approved GLP-1 Receptor Agonists and a Dual GLP-1/GIP Receptor Agonist., PMID:40330819
Higher Compliance with Treatment Administration Instructions for Injectable Dulaglutide versus Oral Semaglutide Reported by People with Type 2 Diabetes in Clinical Practice Settings in Spain: The TRU-Experience Study., PMID:40330535
Tirzepatide Outperforms Dulaglutide for Type 2 Diabetes Control., PMID:40314963
GIPR-Ab/GLP-1 peptide-antibody conjugate requires brain GIPR and GLP-1R for additive weight loss in obese mice., PMID:40301582
Effects of GLP-1 Analogues and Agonists on the Gut Microbiota: A Systematic Review., PMID:40284168
The Potential Role of GLP1-RAs Against Anticancer-Drug Cardiotoxicity: A Scoping Review., PMID:40283534
Exploring Glucagon-Like Peptide-1 Receptor Agonists Usage Among Non-Diabetic Healthcare Providers: A Cross-Sectional Multi-Country Study., PMID:40276133
Clinical Outcomes of Glucagon-Like Peptide-1 Receptor Agonist (GLP-1 RA) and Glucagon-Like Peptide-1/Glucose-Dependent Insulinotropic Polypeptide Receptor Agonist (GLP-1/GIP RA) Exposures Presenting to the Emergency Department., PMID:40269635
Role of Glucagon-Like Peptide-1 on Amyloid, Tau, and α-Synuclein: Target Engagement and Rationale for the Development in Neurodegenerative Disorders., PMID:40252880
Male sexual dysfunction associated with GLP-1 receptor agonists: a cross-sectional analysis of FAERS data., PMID:40240532
Dulaglutide use to improve binge eating behaviors in a person with type 2 diabetes and obesity., PMID:40220283
Comparative evaluation of dulaglutide alone vs. dulaglutide combined with probiotics on cardiovascular risk factors in T2DM., PMID:40214965
Efficacy and Safety of Tirzepatide Compared with GLP-1 RAs in Patients with Type 2 Diabetes Treated with Basal Insulin: A Network Meta-analysis., PMID:40214900
Efficacy and Safety of Dulaglutide Biosimilar LY05008 Versus the Reference Product Dulaglutide (Trulicity) in Chinese Adults With Type 2 Diabetes Mellitus: A Randomized, Open-Label, Active Comparator Study., PMID:40214296
Comparative efficacy and tolerability of currently approved incretin mimetics: A systematic analysis of placebo-controlled clinical trials., PMID:40212008
Unveiling the Therapeutic Potential of Dulaglutide in Mitigating Tacrolimus-Induced Nephrotoxicity Through Targeting the miR-22/HMGB-1/TLR4/MyD88/NF-κB Trajectory., PMID:40205909
Improvement of Glycogenic Hepatopathy With Minimal Corresponding Improvement of Glycemic Control in a Person With Type 1 Diabetes: Case Report and Literature Review., PMID:40201471
A Retrospective Comparative Analysis of Cutaneous Adverse Reactions in GLP-1 Agonist Therapies., PMID:40196945
Comparison of Dose Escalation Versus Switching to Tirzepatide Among People With Type 2 Diabetes Inadequately Controlled on Lower Doses of Dulaglutide : A Randomized Clinical Trial., PMID:40183678
Are Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Central Nervous System (CNS) Penetrant: A Narrative Review., PMID:40172827
Effects of Dulaglutide on Ectopic Fat Deposition in Chronic Kidney Disease (CKD): A Pilot and Feasibility Study (GLIMP)., PMID:40166561
Real world initiation of newly funded empagliflozin and dulaglutide under special authority for patients with type 2 diabetes in New Zealand., PMID:40140836