Catalog No.
DHB74004
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-Lambda
Clonality
Monoclonal
Target
NF-HEV, IL-1F11, C9orf26, IL-33, Interleukin-1 family member 11, IL33, Nuclear factor from high endothelial venules, NFHEV, Interleukin-33, IL1F11
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
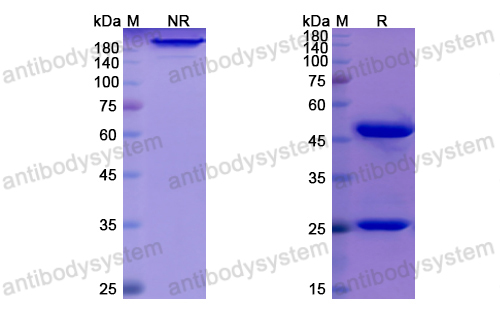
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
O95760
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MEDI-3506, CAS: 2376858-66-9
Clone ID
Tozorakimab
A phase 2a trial of the IL-33 mAb tozorakimab in patients with COPD: FRONTIER-4., PMID:40154559
Efficacy and safety of tozorakimab in moderate-to-severe atopic dermatitis: A Phase 2a randomized controlled trial (FRONTIER-2)., PMID:39535462
Population pharmacokinetic/target engagement modelling of tozorakimab in healthy volunteers and patients with chronic obstructive pulmonary disease., PMID:39183511
Inhibition of Interleukin-33 to Reduce Glomerular Endothelial Inflammation in Diabetic Kidney Disease., PMID:38899206
Towards precision medicine in COPD: Targeting type 2 cytokines and alarmins., PMID:38762432
Advancements in diabetic kidney disease management: integrating innovative therapies and targeted drug development., PMID:38630049
A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose Study to Evaluate the Pharmacokinetics, Immunogenicity, Safety, and Tolerability After Subcutaneous Administration of Tozorakimab in Healthy Chinese Participants., PMID:38523487
A Randomized Phase I Study of the Anti-Interleukin-33 Antibody Tozorakimab in Healthy Adults and Patients With Chronic Obstructive Pulmonary Disease., PMID:38115209
A randomised phase 2a study to investigate the effects of blocking interleukin-33 with tozorakimab in patients hospitalised with COVID-19: ACCORD-2., PMID:37868151
Tozorakimab (MEDI3506): an anti-IL-33 antibody that inhibits IL-33 signalling via ST2 and RAGE/EGFR to reduce inflammation and epithelial dysfunction., PMID:37330528
Real-World Effectiveness of Nirmatrelvir/Ritonavir on Coronavirus Disease 2019-Associated Hospitalization Prevention: A Population-based Cohort Study in the Province of Quebec, Canada., PMID:37149726