Catalog No.
DVV02804
Description
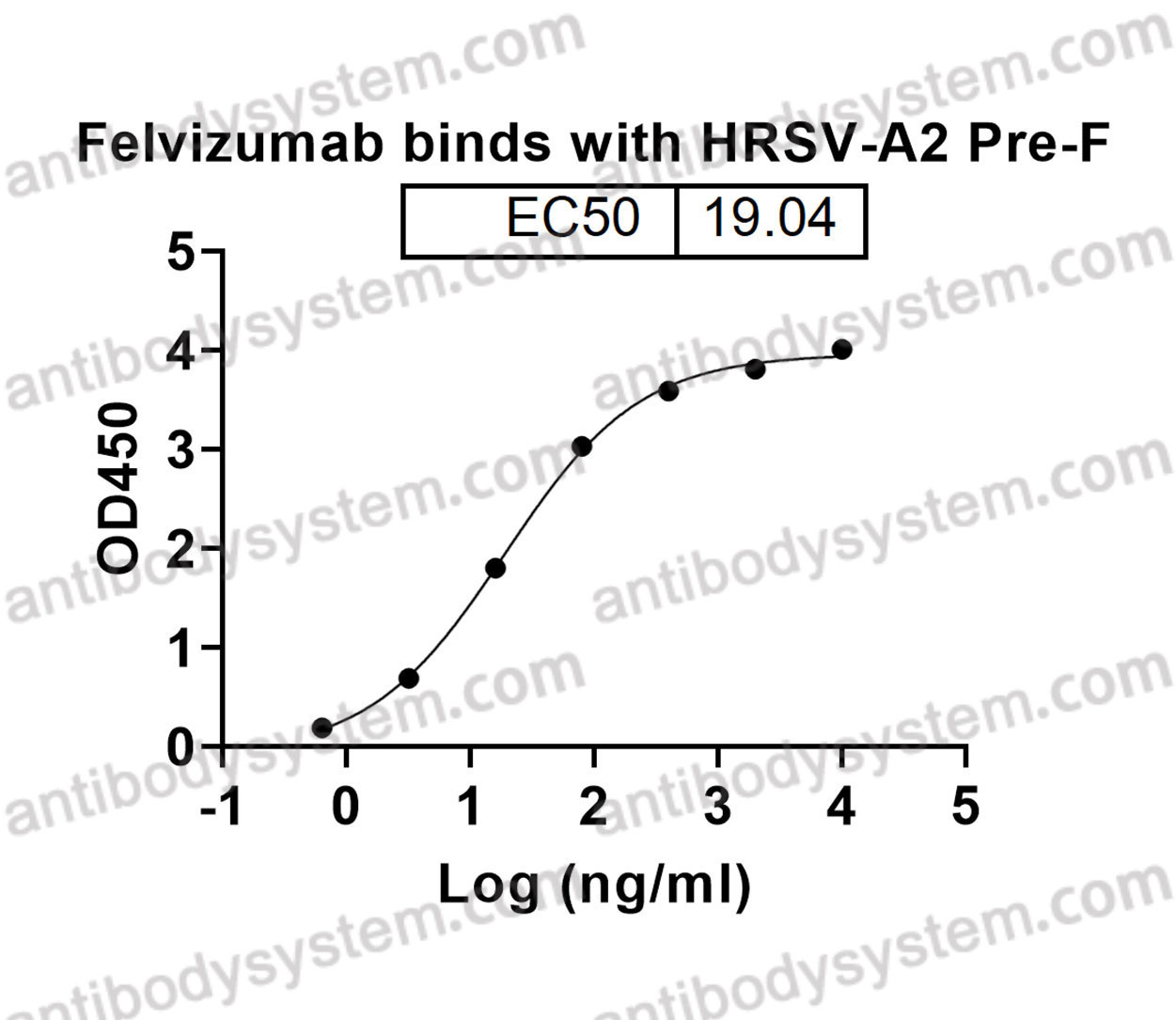
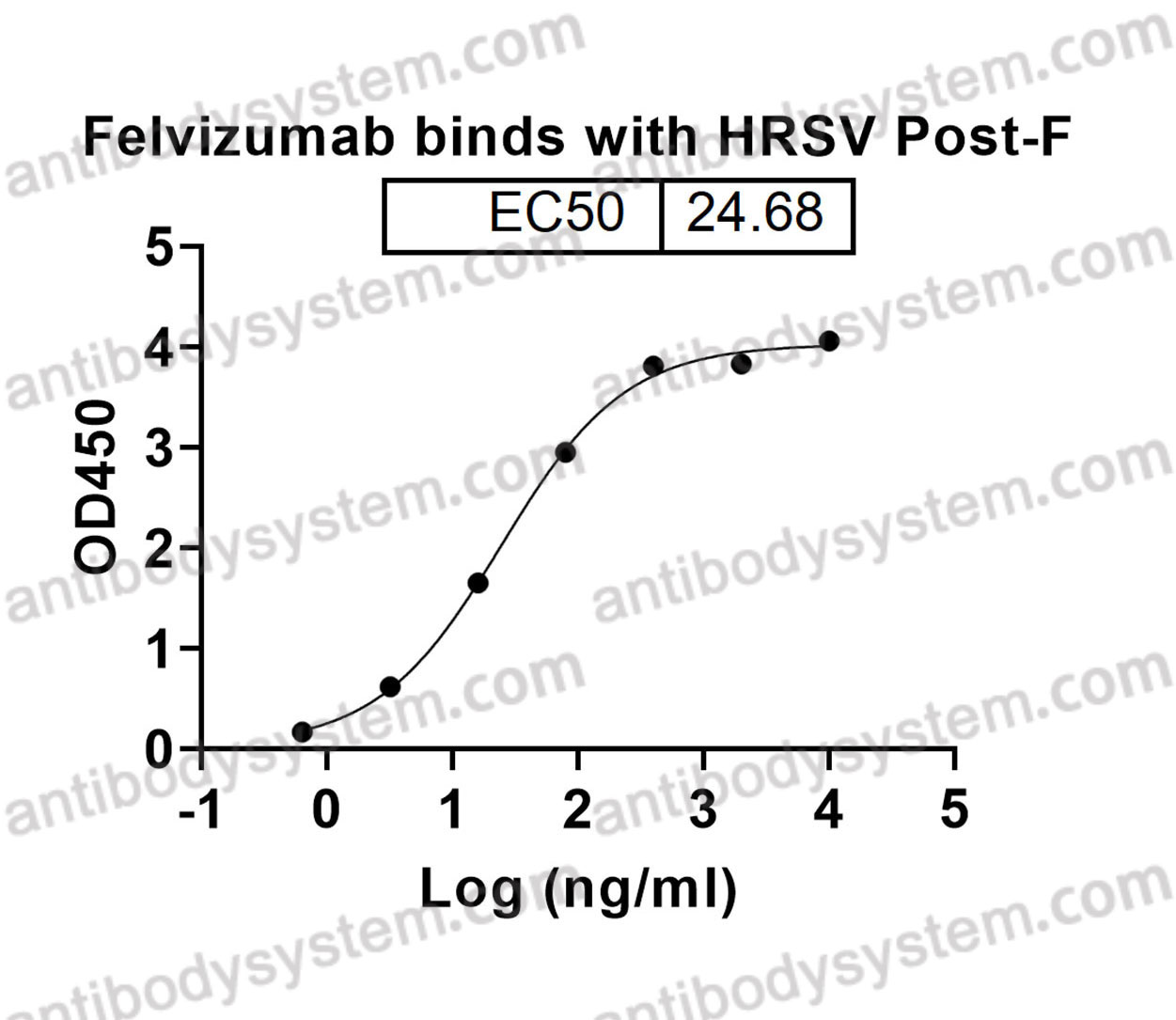
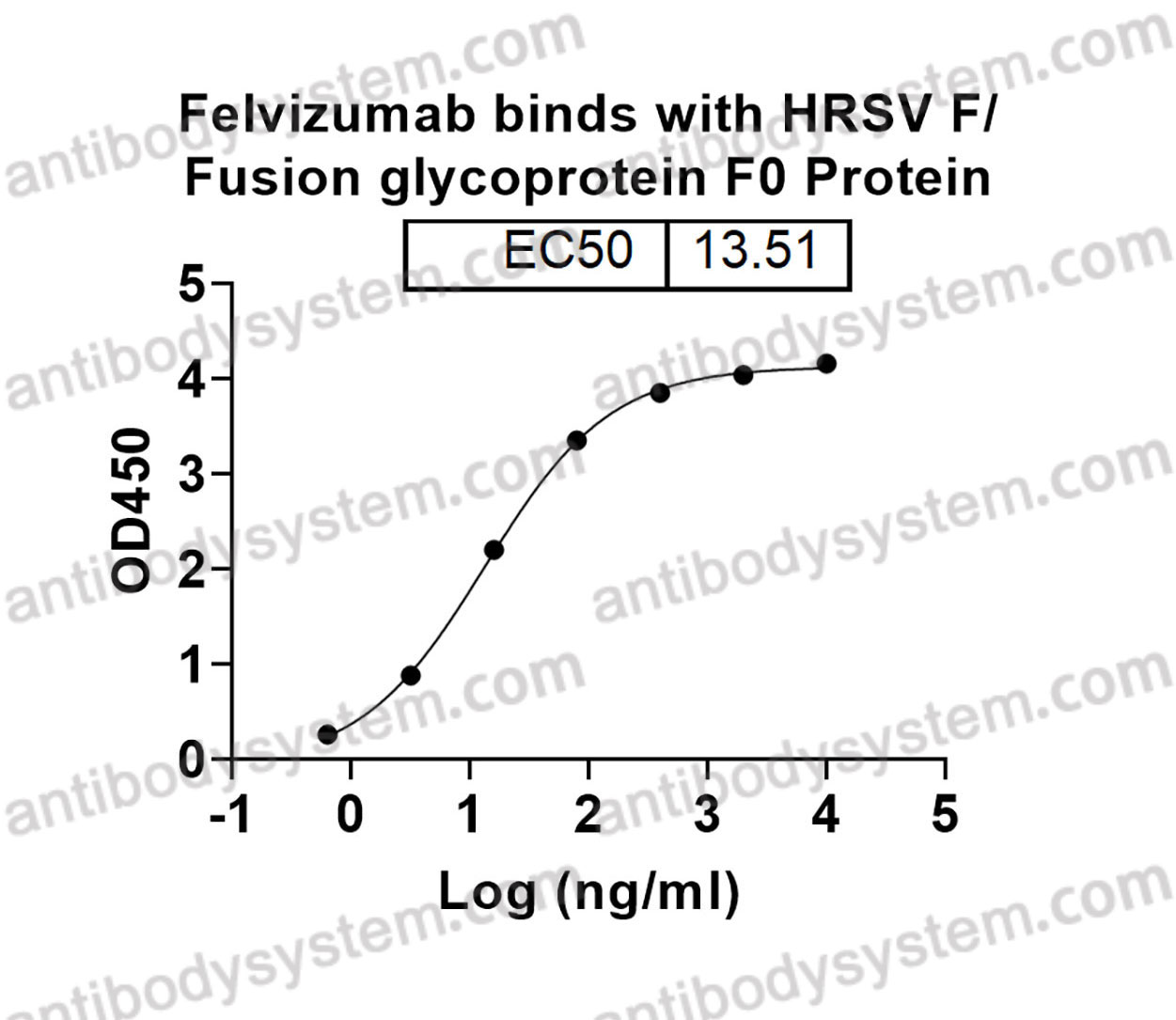
Felvizumab [HuRSV19VHFNS/VK, RSHZ19, RSV monoclonal antibody, SB 209763] is a humanised monoclonal antibody (IgG1) prepared by the humanization of an F protein-specific murine MAb. In in vitro studies, palivizumab, another humanized Mab specific for the F protein of RSV, was 4 to 5-fold more potent than felvizumab in neutralising RSV (Scott, 1999). RSHZ19 (Felvizumab) RSV This antibody was
Expression system
Mammalian Cells
Species reactivity
HRSV-A
Host species
Humanized
Isotype
IgG1, kappa
Clonality
Monoclonal
Target
F, Fusion glycoprotein F0, Fusion glycoprotein F2, p27, Intervening segment, Pep27, Peptide 27, Fusion glycoprotein F1
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
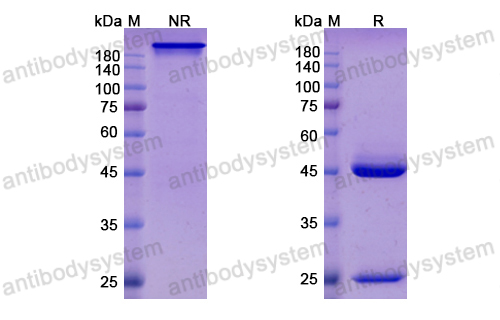
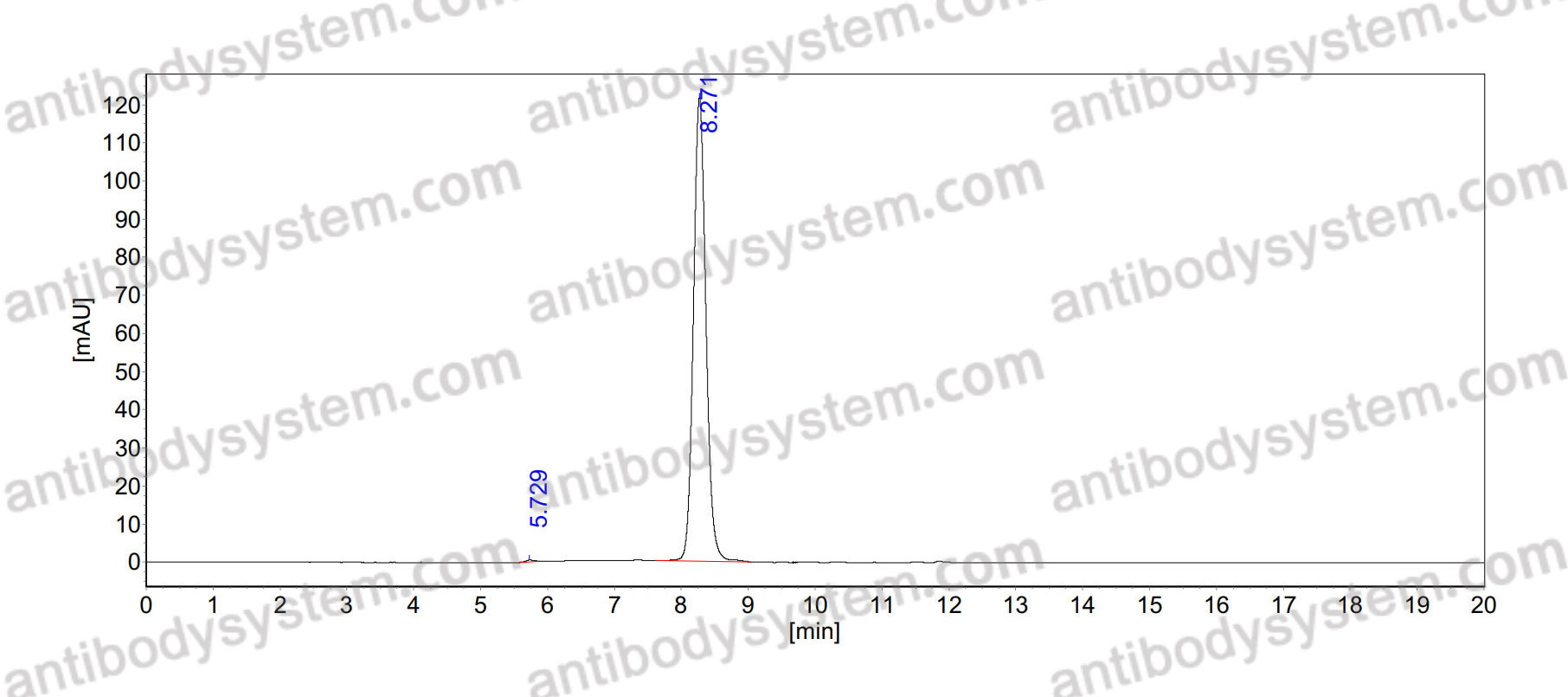
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P03420
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
SB 209763, HuRSV19VHFNS/VK, RSHZ19 monoclonal antibody, SB 209763, CAS: 167747-20-8
Clone ID
Felvizumab
Expert consensus on palivizumab use for respiratory syncytial virus in developed countries, PMID: 31060948
Cost-effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review, PMID: 31040196
Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group, PMID: 9738173
Palivizumab, PMID: 10473022
Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection, PMID: 28605249
Palivizumab: where to from here?, PMID: 19063700
Improving the prescribing of palivizumab, PMID: 29863814
Palivizumab and Long-term Outcomes in Cystic Fibrosis, PMID: 31239289
Palivizumab prophylaxis in preterm infants, PMID: 28219614
Utilization and efficacy of palivizumab for children with Down syndrome, PMID: 31961027
Palivizumab: an overview, PMID: 10707171
Palivizumab's real-world effectiveness: a population-based study in Ontario, Canada, 1993-2017, PMID: 32859612
Adherence and outcomes: a systematic review of palivizumab utilization, PMID: 29130355
Palivizumab Prophylaxis and Recurrent Wheezing, PMID: 29019698
Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis, PMID: 23737087
Respiratory Syncytial Virus Bronchiolitis in Children, PMID: 28084708
Initial Palivizumab Dose Administration in Outpatient Clinic After Hospital Discharge, PMID: 29570593
Neutralizing epitopes of RSV and palivizumab resistance in Japan, PMID: 28867684
Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis, PMID: 22336832
Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis, PMID: 24851825
Universal palivizumab prophylaxis for children with Down syndrome in Japan: analysis with interrupted time-series, PMID: 32961094
Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis, PMID: 27439110
Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis, PMID: 20166098
Palivizumab Following Extremely Premature Birth Does Not Affect Pulmonary Outcomes in Adolescence, PMID: 32298728
Palivizumab for respiratory syncytial virus prophylaxis, PMID: 10690084
Administration of Palivizumab in the NICU, PMID: 27164941
Palivizumab prophylaxis in 'late preterm' newborns, PMID: 20695756
Is palivizumab effective as a prophylaxis of respiratory syncytial virus infections in cystic fibrosis patients? A meta-analysis, PMID: 24231153
Consensus conference on the appropriateness of palivizumab prophylaxis in respiratory syncytial virus disease, PMID: 27618642
Effectiveness of Palivizumab against Respiratory Syncytial Virus: Cohort and Case Series Analysis, PMID: 31378522
A review of palivizumab and emerging therapies for respiratory syncytial virus, PMID: 21831008
Reducing Palivizumab Dose Requirements Through Rational Dose Regimen Design, PMID: 30426719
Optimizing Lung Function in Survivors of Preterm Birth: Palivizumab Is Not the Answer, PMID: 32768062
Systematic Review of the Safety and Efficacy of Palivizumab among Infants and Young Children with Cystic Fibrosis, PMID: 28423192
Treatment of respiratory syncytial virus with palivizumab: a systematic review, PMID: 21080142
The potential impact of palivizumab on pediatric airway reconstruction, PMID: 16360816
Bronchiolitis: an update on management and prophylaxis, PMID: 31059347
Palivizumab and prevention of childhood respiratory syncytial viral infection: protocol for a systematic review and meta-analysis of breakthrough infections, PMID: 31340973
Palivizumab administration in preterm infants in France: EPIPAGE-2 cohort study, PMID: 29395887
Palivizumab prophylaxis, respiratory syncytial virus and subsequent development of asthma, PMID: 29795072
A review of cost-effectiveness of palivizumab for respiratory syncytial virus, PMID: 23140255
Palivizumab use in infants with Down syndrome-report from the German Synagis™ Registry 2009-2016, PMID: 29651734
Palivizumab in the prophylaxis of respiratory syncytial virus infection, PMID: 16207163
Restricted Palivizumab Recommendations and the Impact on RSV Hospitalizations among Infants Born at > 29 Weeks of Gestational Age: An Italian Multicenter Study, PMID: 31238365
Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV), PMID: 26670908
Palivizumab and the prevention of respiratory syncytial virus illness in pediatric patients with congenital heart disease, PMID: 17727335
Role of viral infections in the development and exacerbation of asthma in children, PMID: 28987219
Palivizumab prophylaxis of respiratory syncytial virus infection in high-risk infants, PMID: 12410864
The cost effectiveness of palivizumab: a systematic review of the evidence, PMID: 20653398
Palivizumab Prophylaxis Against Respiratory Syncytial Virus Infection in Children with Immunocompromised Conditions or Down Syndrome: A Multicenter, Post-Marketing Surveillance in Japan, PMID: 28895096