Catalog No.
DVV03804
Expression system
Mammalian Cells
Species reactivity
Influenza A virus
Host species
Human
Isotype
IgG1, kappa
Clonality
Monoclonal
Target
HA/Hemagglutinin
Concentration
1.4 mg/ml
Endotoxin level
Please contact with the lab for this information.
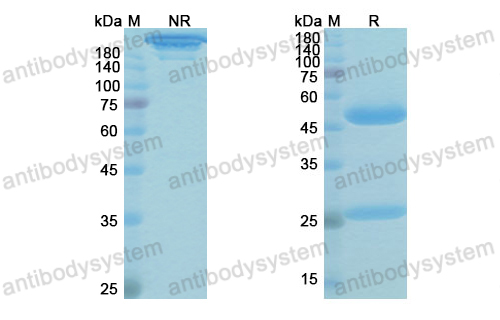
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
C6KNH7
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MHAA4549A, RO6876802, RG7745, CAS: 1807954-17-1
Clone ID
Gedivumab
Pharmacokinetics of the Monoclonal Antibody MHAA4549A Administered in Combination With Oseltamivir in Patients Hospitalized With Severe Influenza A Infection, PMID: 32621543
A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of MHAA4549A, a Monoclonal Antibody, plus Oseltamivir in Patients Hospitalized with Severe Influenza A Virus Infection, PMID: 32393496
Pharmacokinetics of MHAA4549A, an Anti-Influenza A Monoclonal Antibody, in Healthy Subjects Challenged with Influenza A Virus in a Phase IIa Randomized Trial, PMID: 28639229
Pharmacokinetics of the Monoclonal Antibody MHAA4549A Administered in Combination With Oseltamivir in Patients Hospitalized With Severe Influenza A Infection., PMID:32621543
A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of MHAA4549A, a Monoclonal Antibody, plus Oseltamivir in Patients Hospitalized with Severe Influenza A Virus Infection., PMID:32393496
Pharmacokinetics of MHAA4549A, an Anti-Influenza A Monoclonal Antibody, in Healthy Subjects Challenged with Influenza A Virus in a Phase IIa Randomized Trial., PMID:28639229