Catalog No.
DVV03801
Description
Diridavumab, the alternative names CR6261, is a human monoclonal antibody direct against influenza A hemagglutinin. The CR6261 antibody was first discovered by the Scripps Institute and Crucell, a dutch biopharmaceutical company, and later developed by Janssen Pharmaceutical Companies of Johnson & Johnson under the trade name diridavumab.
Cr6261 is under investigation in clinical trial NCT02016066 (A Study to Assess the Safety, Pharmacokinetics and Immunogenicity of Diridavumab in Japanese Healthy Participants).
Expression system
Mammalian Cells
Species reactivity
Influenza A virus
Host species
Human
Isotype
IgG1, lambda
Clonality
Monoclonal
Target
HA/Hemagglutinin
Concentration
1.66 mg/ml
Endotoxin level
Please contact with the lab for this information.
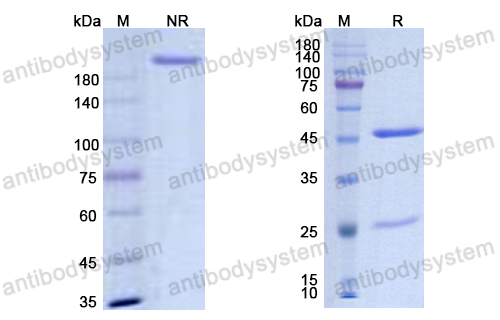
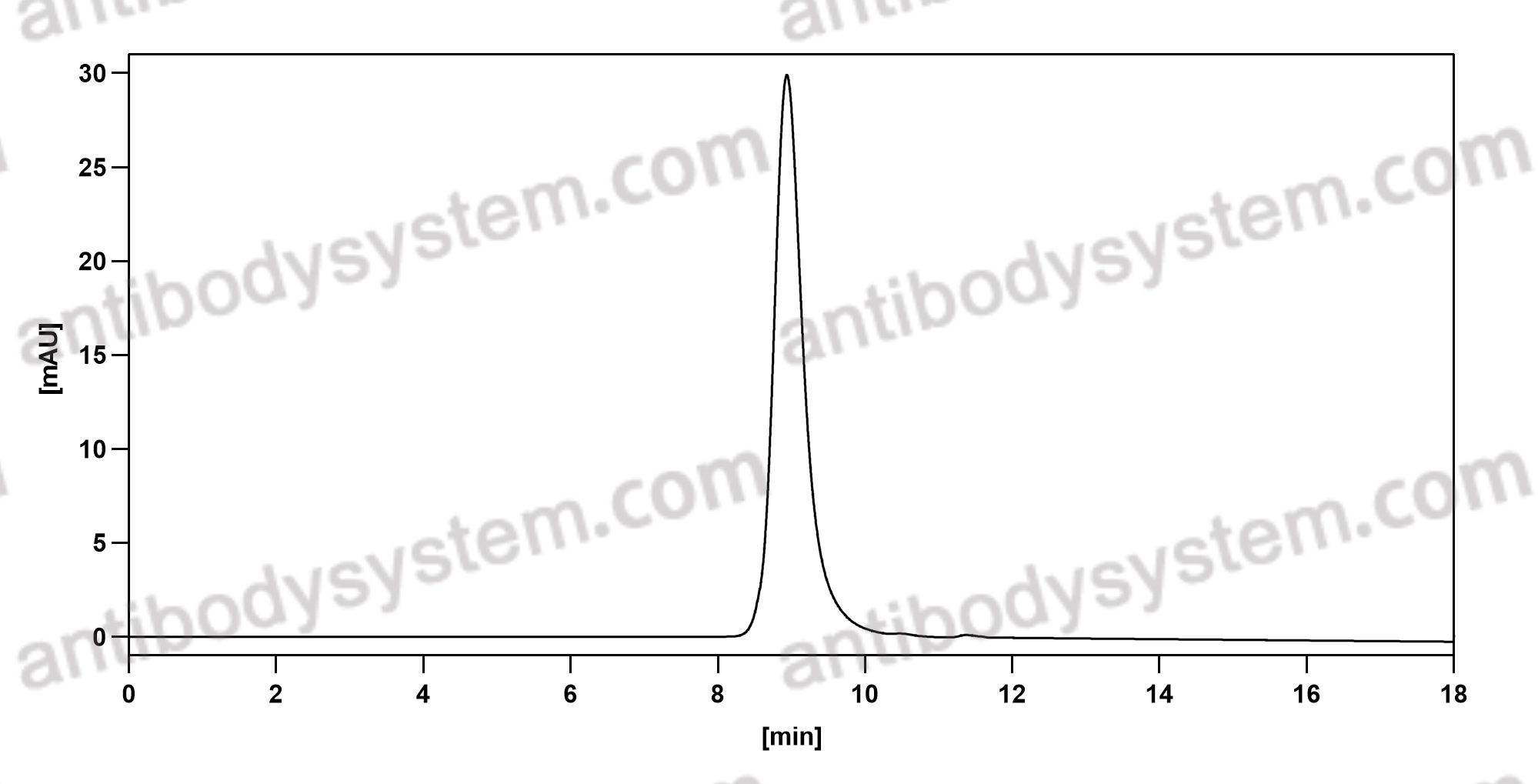
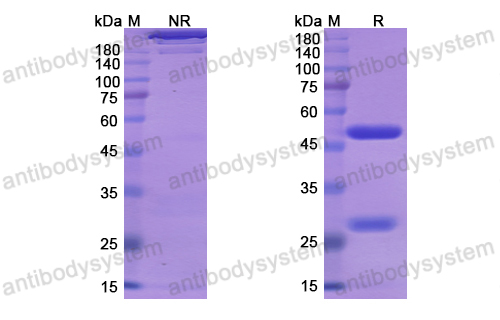
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P03455
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CR-6261, CR6261, CAS: 1393659-46-5
Clone ID
Diridavumab
Safety and Efficacy of CR6261 in an Influenza A H1N1 Healthy Human Challenge Model. PMID: 33211860
Antibody recognition of a highly conserved influenza virus epitope. PMID: 19251591
The molecular mechanism of action of the CR6261-Azichromycin combination found through computational analysis. PMID: 22693576
In Vitro Neutralization Is Not Predictive of Prophylactic Efficacy of Broadly Neutralizing Monoclonal Antibodies CR6261 and CR9114 against Lethal H2 Influenza Virus Challenge in Mice. PMID: 29046448
Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins. PMID: 33049994
[Effect of antibody CR6261 V(H) and scFv expression on influenza virus infection]. PMID: 22260064
Binding affinity landscapes constrain the evolution of broadly neutralizing anti-influenza antibodies. PMID: 34491198
Discovery of protective B-cell epitopes for development of antimicrobial vaccines and antibody therapeutics. PMID: 24219801
Cross-neutralizing Anti-hemagglutinin Antibodies Isolated from Patients Infected with Avian Influenza A (H5N1) Virus. PMID: 32131957
Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PMID: 19079604
A small-molecule fusion inhibitor of influenza virus is orally active in mice. PMID: 30846569
Monoclonal Antibody Purification (Nicotiana benthamiana Plants). PMID: 27547788
New class of monoclonal antibodies against severe influenza: prophylactic and therapeutic efficacy in ferrets. PMID: 20161706
Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. PMID: 24344259
Structural and genetic basis for development of broadly neutralizing influenza antibodies. PMID: 22932267
Design of Escherichia coli-expressed stalk domain immunogens of H1N1 hemagglutinin that protect mice from lethal challenge. PMID: 23015722
Pre- and postexposure use of human monoclonal antibody against H5N1 and H1N1 influenza virus in mice: viable alternative to oseltamivir. PMID: 19911992
Unmasking Stem-Specific Neutralizing Epitopes by Abolishing N-Linked Glycosylation Sites of Influenza Virus Hemagglutinin Proteins for Vaccine Design. PMID: 27440889
A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. PMID: 22896619
Cross-Neutralising Nanobodies Bind to a Conserved Pocket in the Hemagglutinin Stem Region Identified Using Yeast Display and Deep Mutational Scanning. PMID: 27741319
Exploring the early stages of the pH-induced conformational change of influenza hemagglutinin. PMID: 24854389
Relating influenza virus membrane fusion kinetics to stoichiometry of neutralizing antibodies at the single-particle level. PMID: 25404330
Broadly Reactive Human Monoclonal Antibodies Elicited following Pandemic H1N1 Influenza Virus Exposure Protect Mice against Highly Pathogenic H5N1 Challenge. PMID: 29899095
Free-energy simulations reveal that both hydrophobic and polar interactions are important for influenza hemagglutinin antibody binding. PMID: 22455929
Glycan masking of hemagglutinin for adenovirus vector and recombinant protein immunizations elicits broadly neutralizing antibodies against H5N1 avian influenza viruses. PMID: 24671139
HA Antibody-Mediated FcγRIIIa Activity Is Both Dependent on FcR Engagement and Interactions between HA and Sialic Acids. PMID: 27746785