Catalog No.
DVV05101
Expression system
Mammalian Cells
Species reactivity
Human cytomegalovirus (HCMV/HHV-5)
Host species
Human
Isotype
IgG1, kappa
Clonality
Monoclonal
Target
Envelope glycoprotein H, gH, gH, UL75
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
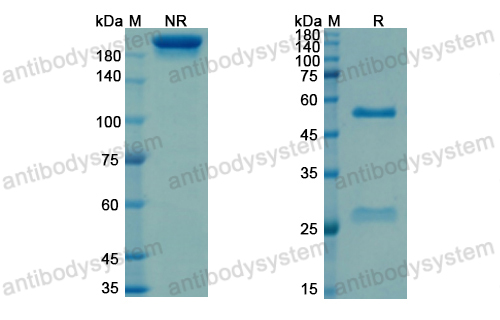
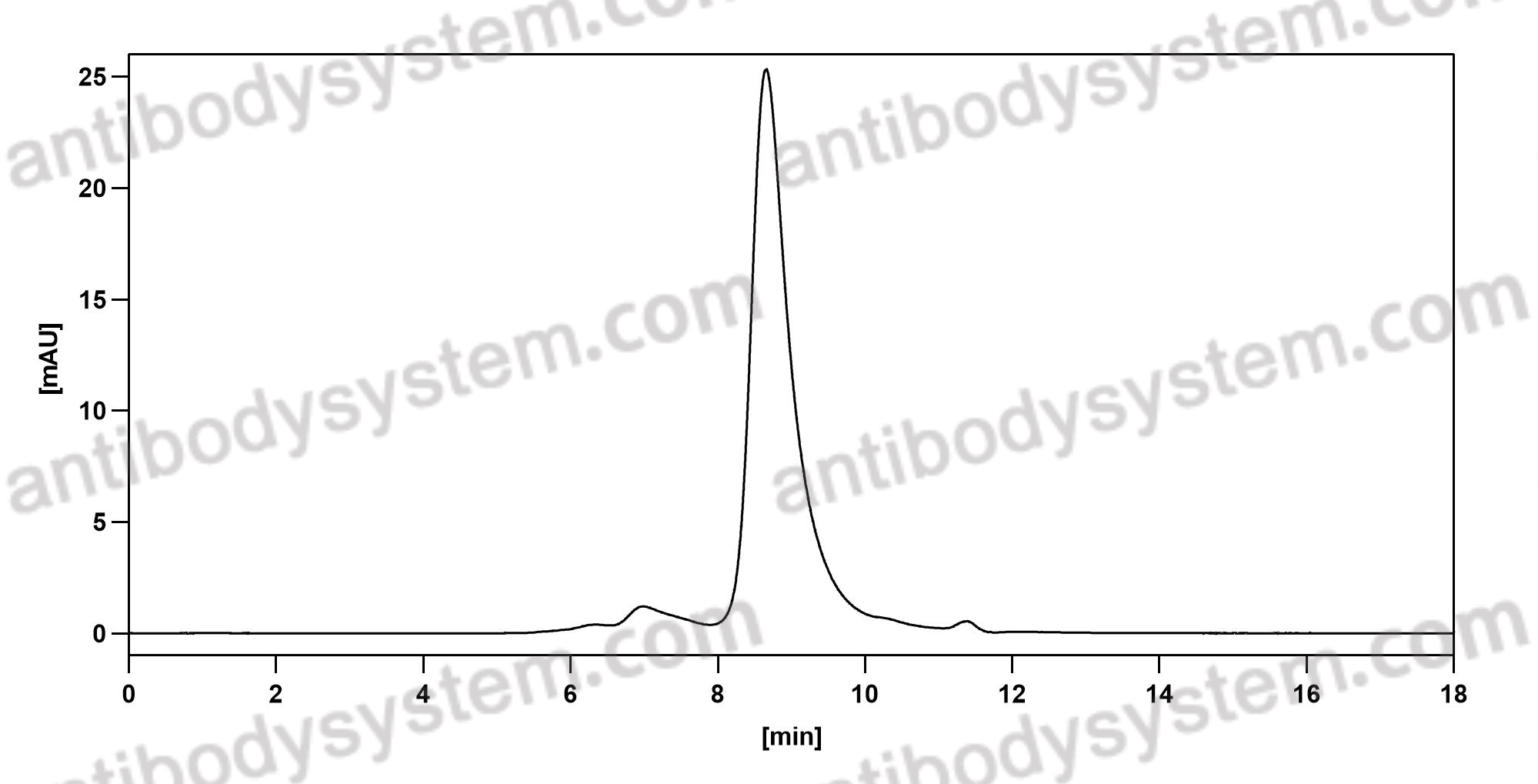
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P12824
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
EV2 7, MSL 109, SDZ MSL 109, CAS: 138660-96-5
Clone ID
Sevirumab
Sevirumab. Protovir, MSL 109, EV2 7, SDZ MSL 109, PMID: 10728470
Therapeutic developments in cytomegalovirus retinitis, PMID: 11060672
Current antiviral strategies for controlling cytomegalovirus in hematopoietic stem cell transplant recipients: prevention and therapy, PMID: 11428987
Mechanism for neutralizing activity by the anti-CMV gH/gL monoclonal antibody MSL-109, PMID: 24843144
In vitro affinity maturation of a natural human antibody overcomes a barrier to in vivo affinity maturation, PMID: 24492299
Visual loss in patients with cytomegalovirus retinitis and acquired immunodeficiency syndrome before widespread availability of highly active antiretroviral therapy, PMID: 12523893
A phase II, double-masked, randomized, placebo-controlled evaluation of a human monoclonal anti-Cytomegalovirus antibody (MSL-109) in combination with standard therapy versus standard therapy alone in the treatment of AIDS patients with Cytomegalovirus retinitis, PMID: 15498605
HIV and cytomegalovirus viral load and clinical outcomes in AIDS and cytomegalovirus retinitis patients: Monoclonal Antibody Cytomegalovirus Retinitis Trial, PMID: 11919489
Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation, PMID: 11464977
Data and safety monitoring board deliberations resulting in the early termination of the Monoclonal Antibody Cytomegalovirus Retinitis Trial, PMID: 12559647
Mechanism for neutralizing activity by the anti-CMV gH/gL monoclonal antibody MSL-109., PMID:24843144
In vitro affinity maturation of a natural human antibody overcomes a barrier to in vivo affinity maturation., PMID:24492299
A phase II, double-masked, randomized, placebo-controlled evaluation of a human monoclonal anti-Cytomegalovirus antibody (MSL-109) in combination with standard therapy versus standard therapy alone in the treatment of AIDS patients with Cytomegalovirus retinitis., PMID:15498605
Data and safety monitoring board deliberations resulting in the early termination of the Monoclonal Antibody Cytomegalovirus Retinitis Trial., PMID:12559647
Visual loss in patients with cytomegalovirus retinitis and acquired immunodeficiency syndrome before widespread availability of highly active antiretroviral therapy., PMID:12523893
HIV and cytomegalovirus viral load and clinical outcomes in AIDS and cytomegalovirus retinitis patients: Monoclonal Antibody Cytomegalovirus Retinitis Trial., PMID:11919489
Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation., PMID:11464977
Current antiviral strategies for controlling cytomegalovirus in hematopoietic stem cell transplant recipients: prevention and therapy., PMID:11428987
Therapeutic developments in cytomegalovirus retinitis., PMID:11060672
Sevirumab. Protovir, MSL 109, EV2 7, SDZ MSL 109., PMID:10728470