Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, PMID: 33475701
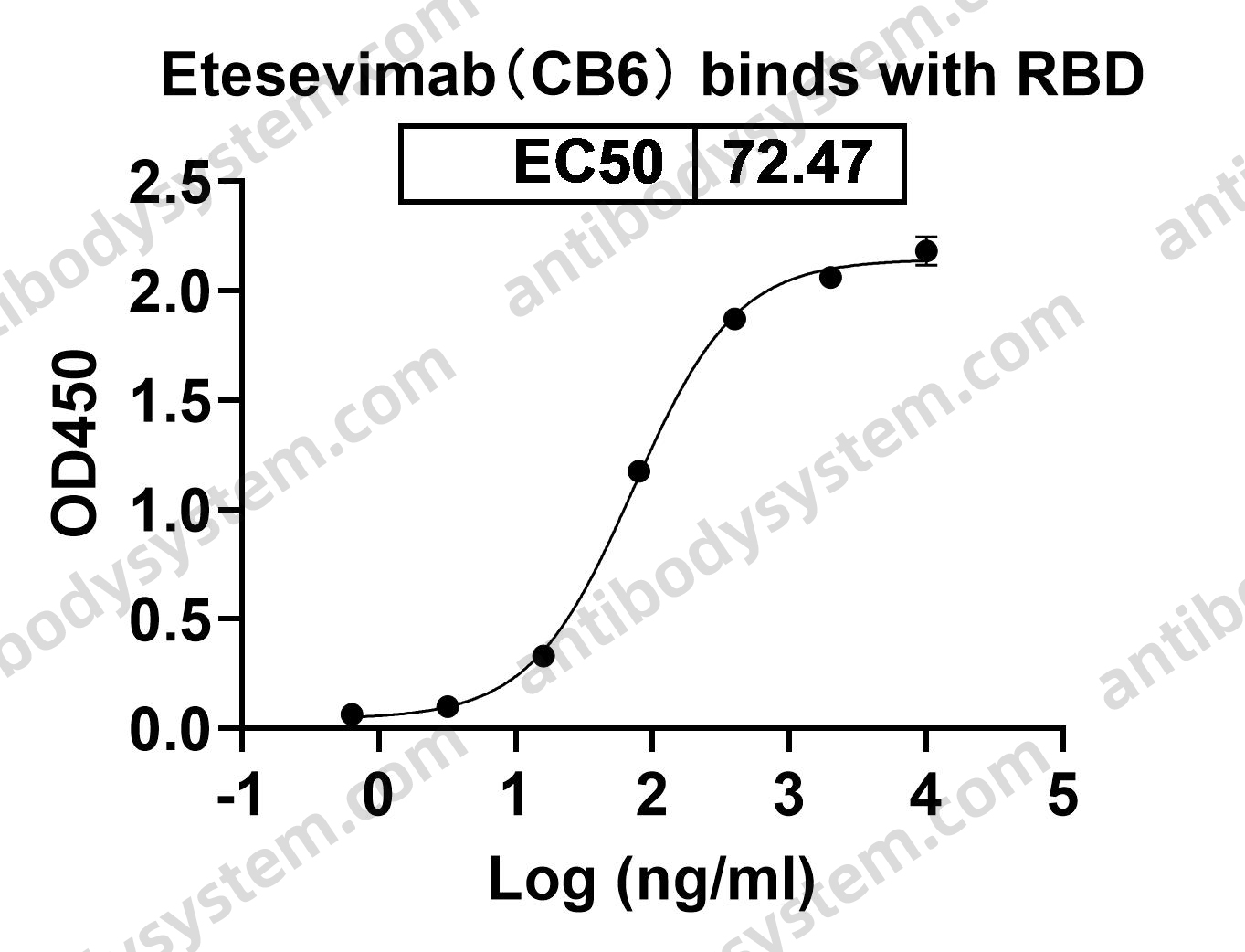
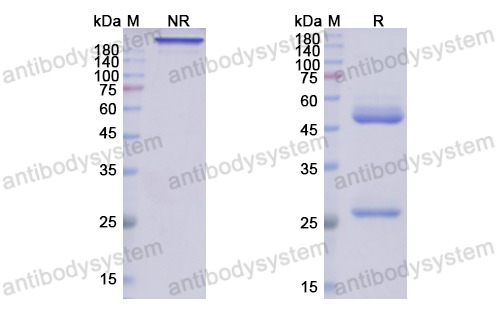
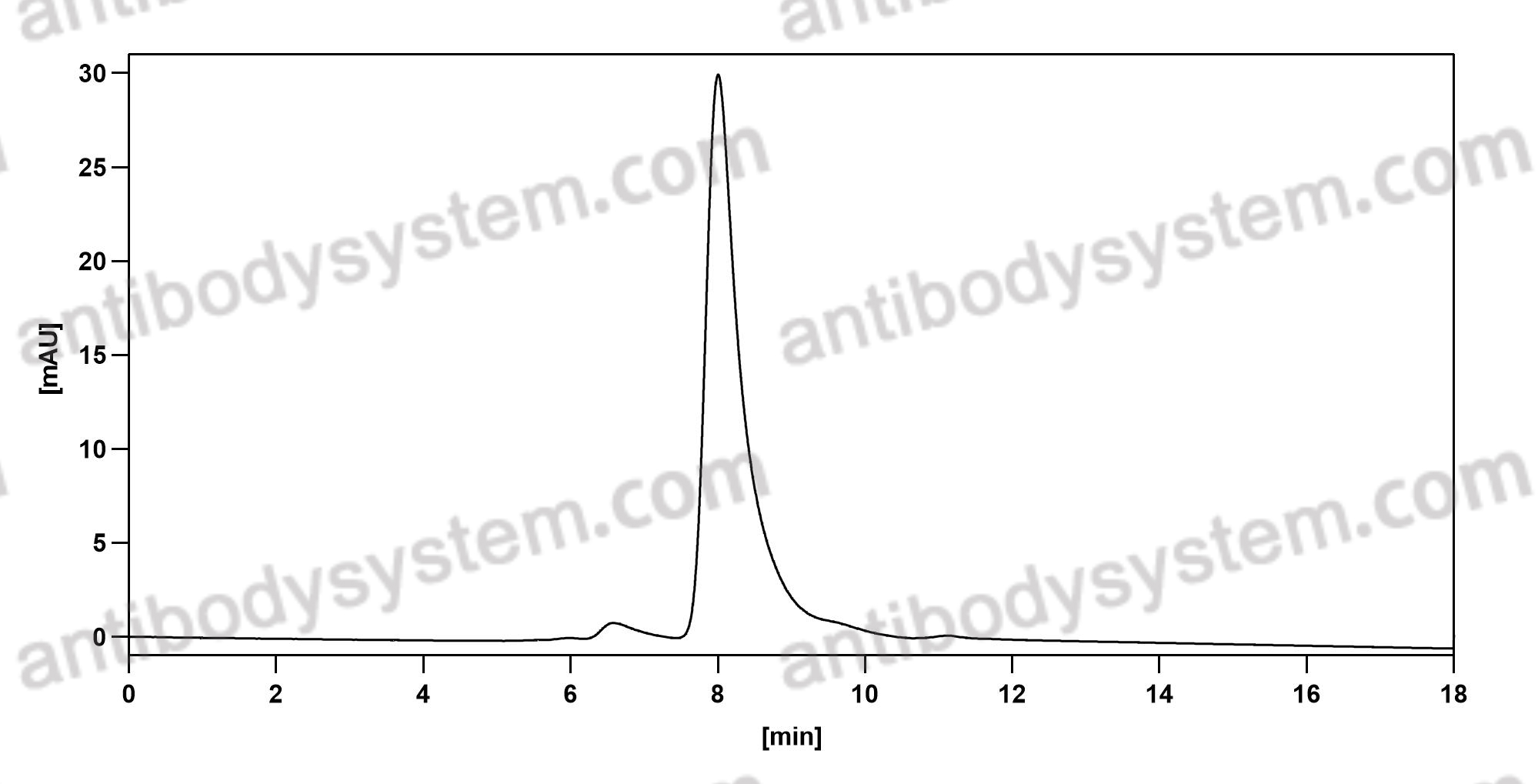
Etesevimab, PMID: 34283469
Bamlanivimab, PMID: 33226744
Etesevimab and Bamlanivimab, PMID: 33630482
Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, PMID: 34260849
A Narrative Review of the Clinical Practicalities of Bamlanivimab and Etesevimab Antibody Therapies for SARS-CoV-2, PMID: 34374951
An EUA for bamlanivimab and etesevimab for COVID-19, PMID: 33830966
Tolerability, Safety, Pharmacokinetics, and Immunogenicity of a Novel SARS-CoV-2 Neutralizing Antibody, Etesevimab, in Chinese Healthy Adults: a Randomized, Double-Blind, Placebo-Controlled, First-in-Human Phase 1 Study, PMID: 33972256
Bamlivimab + etesevimab therapy induces SARS-COV-2 immune escape mutations and secondary clinical deterioration in Covid-19 patients with B cell malignancies, PMID: 34352377
Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern, PMID: 34435337
Emergence of Q493R mutation in SARS-CoV-2 spike protein during bamlanivimab/etesevimab treatment and resistance to viral clearance, PMID: 34437928
Neutralizing Antibody Therapeutics for COVID-19, PMID: 33916927
Development and application of therapeutic antibodies against COVID-19, PMID: 33907512
Initial experience of bamlanivimab monotherapy use in solid organ transplant recipients, PMID: 34081820
Monoclonal Antibody Therapy in a Vaccine Breakthrough SARS-CoV-2 Hospitalized Delta (B.1.617.2) Variant Case, PMID: 34271202
Editorial: Post-Exposure Prophylactic Neutralizing Monoclonal Antibodies to SARS-CoV-2 for Individuals at High Risk for COVID-19, PMID: 34393218
Perspectives on passive antibody therapy and peptide-based vaccines against emerging pathogens like SARS-CoV-2, PMID: 34422699
Post Acute Coronavirus (COVID-19) Syndrome, PMID: 34033370
Interventions in an Ambulatory Setting to Prevent Progression to Severe Disease in Patients With COVID-19: A Systematic Review, PMID: 34157890
Implementing a method for engineering multivalency to substantially enhance binding of clinical trial anti-SARS-CoV-2 antibodies to wildtype spike and variants of concern proteins, PMID: 34006961
An EUA for sotrovimab for treatment of COVID-19, PMID: 34181630
Update: Drug treatment options for coronavirus disease 2019 (COVID-19), PMID: 34433754
COVID antibody treatments show promise for preventing severe disease, PMID: 33712752
SARS-CoV-2 Variants: A Synopsis of In Vitro Efficacy Data of Convalescent Plasma, Currently Marketed Vaccines, and Monoclonal Antibodies, PMID: 34201767
Monoclonal Antibodies: Medical Uses for the Prevention and Treatment of Disease, PMID: 33861166
Prospective mapping of viral mutations that escape antibodies used to treat COVID-19, PMID: 33495308
An overview of the preclinical discovery and development of bamlanivimab for the treatment of novel coronavirus infection (COVID-19): reasons for limited clinical use and lessons for the future, PMID: 34304682
Emergence of SARS-COV-2 Spike Protein Escape Mutation Q493R after Treatment for COVID-19, PMID: 34314668
Monoclonal Antibodies for Early Treatment of COVID-19 in a World of Evolving SARS-CoV-2 Mutations and Variants, PMID: 34291118
Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines, PMID: 33724631
The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization, PMID: 34034784
Effects of the glycosylation of the receptor binding domain (RBD dimer)-based Covid-19 vaccine (ZF2001) on its humoral immunogenicity and immunoreactivity. [DVV00306]
Effectiveness of Anti-SARS-CoV-2 monoclonal antibodies in real-life: RNAemia and clinical outcomes in high-risk COVID-19 patients., PMID:40279347
Innate and SARS-CoV-2 specific adaptive immune response kinetic in neutralizing monoclonal antibody successfully treated COVID-19 patients., PMID:39832460
Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: A Multicentric Retrospective Study., PMID:39459877
Adverse events associated with SARS-CoV-2 neutralizing monoclonal antibodies using the FDA adverse event reporting system database., PMID:39345748
Impact analysis of SARS-CoV-2 vaccination in patients treated with monoclonal antibodies: A monocentric experience., PMID:39265354
Monoclonal antibodies against SARS-CoV-2 to prevent COVID-19 worsening in a large multicenter cohort., PMID:39247344
Efficacy and Safety of Low-Dose, Rapidly Infused Bamlanivimab and Etesevimab: Phase 3 BLAZE-1 Trial for Mild-to-Moderate COVID-19., PMID:39230829
Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities., PMID:39126484
Clinical outcomes in patients with mild to moderate coronavirus disease 2019 treated with monoclonal antibody therapy versus an untreated control cohort., PMID:39066463
Sotrovimab in the treatment of coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis of randomized clinical trials., PMID:39031183
Comparison of bamlanivimab with or without etesevimab and casirivimab-imdevimab in clinical outcomes in patients with COVID-19: a systematic review and meta-analysis., PMID:38721976
Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab or Sotrovimab on Persistently High Viral Load in Patients with Mild-to-Moderate COVID-19: A Randomized, Phase 2 BLAZE-4 Trial., PMID:38291279
Effects of N-glycan modifications on spike expression, virus infectivity, and neutralization sensitivity in ancestral compared to Omicron SARS-CoV-2 variants., PMID:37943965
The Efficacy and Safety of Monoclonal Antibody Treatments Against COVID-19: A Systematic Review and Meta-analysis of Randomized Clinical Trials., PMID:37915159
Assessment of neutralization susceptibility of Omicron subvariants XBB.1.5 and BQ.1.1 against broad-spectrum neutralizing antibodies through epitopes mapping., PMID:37828918
Antiviral Drugs and Vaccines for Omicron Variant: A Focused Review., PMID:37719798
A Comparison of the Adverse Effects and Utility of Different Monoclonal Antibodies for SARS-CoV-2: A Retrospective Cohort Study., PMID:37680398
Use of Monoclonal Antibodies in Pregnant Women Infected by COVID-19: A Case Series., PMID:37630512
Low BAU/ml values with 4 of 5 SARS CoV-2 spike-specific monoclonal antibodies in the Roche Elecsys antibody assay., PMID:37516369
Preliminary Evidence of Good Safety Profile and Outcomes of Early Treatment with Tixagevimab/Cilgavimab Compared to Previously Employed Monoclonal Antibodies for COVID-19 in Immunocompromised Patients., PMID:37371635
Pharmacokinetics, Efficacy, and Safety of a SARS-CoV-2 Antibody Treatment in Pediatric Participants: An Open-Label Addendum of a Placebo-Controlled, Randomized Phase 2/3 Trial., PMID:37329415
Neutralizing anti-spike monoclonal antibodies for COVID-19 in vulnerable populations: lessons learned and future directions., PMID:37318043
Effects of bamlanivimab alone or in combination with etesevimab on subsequent hospitalization and mortality in outpatients with COVID-19: a systematic review and meta-analysis., PMID:37180576
Efficacy and Safety of Anti-SARS-CoV-2 Monoclonal Antibodies: An Updated Review., PMID:37129306
Neutralizing Monoclonal Antibody Use and COVID-19 Infection Outcomes., PMID:37093599
Rational strategies for enhancing mAb binding to SARS-CoV-2 variants through CDR diversification and antibody-escape prediction., PMID:37063859
Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 : A Cohort Study., PMID:37011399
Insights on the interaction of SARS-CoV-2 variant B.1.617.2 with antibody CR3022 and analysis of antibody resistance., PMID:36940010
Monoclonal Antibody Use for Coronavirus Disease 2019 in Pediatric Patients: A Multicenter Retrospective Study., PMID:36928172
Lung Ultrasound Is Useful for Evaluating Lung Damage in COVID-19 Patients Treated with Bamlanivimab and Etesevimab: A Single-Center Pilot Study., PMID:36837405
Monoclonal Antibody Therapy for COVID-19: A Retrospective Observational Study at a Regional Hospital., PMID:36826353
Monoclonal antibody infusion reaction with bamlanivimab and etesevimab in a 5-year-old male with coronavirus disease 2019: a case report., PMID:36823658
Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments., PMID:36727404
The evidence base for emergency use authorizations for COVID-19 treatments: A rapid review., PMID:36644312
Circulating microRNAs as emerging regulators of COVID-19., PMID:36593971
Cardiovascular Adverse Events Associated with Monoclonal Antibody Products in Patients with COVID-19., PMID:36558922
A review of COVID-19 therapeutics in pregnancy and lactation., PMID:36514791
Cross-reaction of current available SARS-CoV-2 MAbs against the pangolin-origin coronavirus GX/P2V/2017., PMID:36493785
Increased reporting of venous and arterial thromboembolic events reported with tixagevimab-cilgavimab for coronavirus disease 2019., PMID:36464214
Investigation of SARS-CoV-2 Variants and Their Effect on SARS-CoV-2 Monoclonal Antibodies, Convalescent and Vaccine Plasma by a Novel Web Tool., PMID:36428929
Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern., PMID:36413383
Monoclonal Antibodies for Treatment of SARS-CoV-2 Infection During Pregnancy : A Cohort Study., PMID:36375150
Monoclonal Antibodies against SARS-CoV-2 Infection: Results from a Real-Life Study before the Omicron Surge., PMID:36366403
A Comprehensive Review on the Efficacy of Several Pharmacologic Agents for the Treatment of COVID-19., PMID:36362912
Antispike monoclonal antibodies for prevention and treatment of coronavirus disease-2019 in solid organ transplant recipients., PMID:36354253
Comparative effectiveness of neutralising monoclonal antibodies in high risk COVID-19 patients: a Bayesian network meta-analysis., PMID:36266486