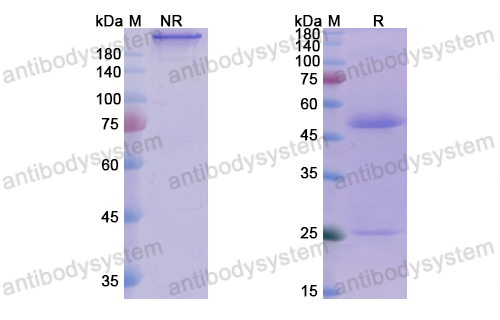
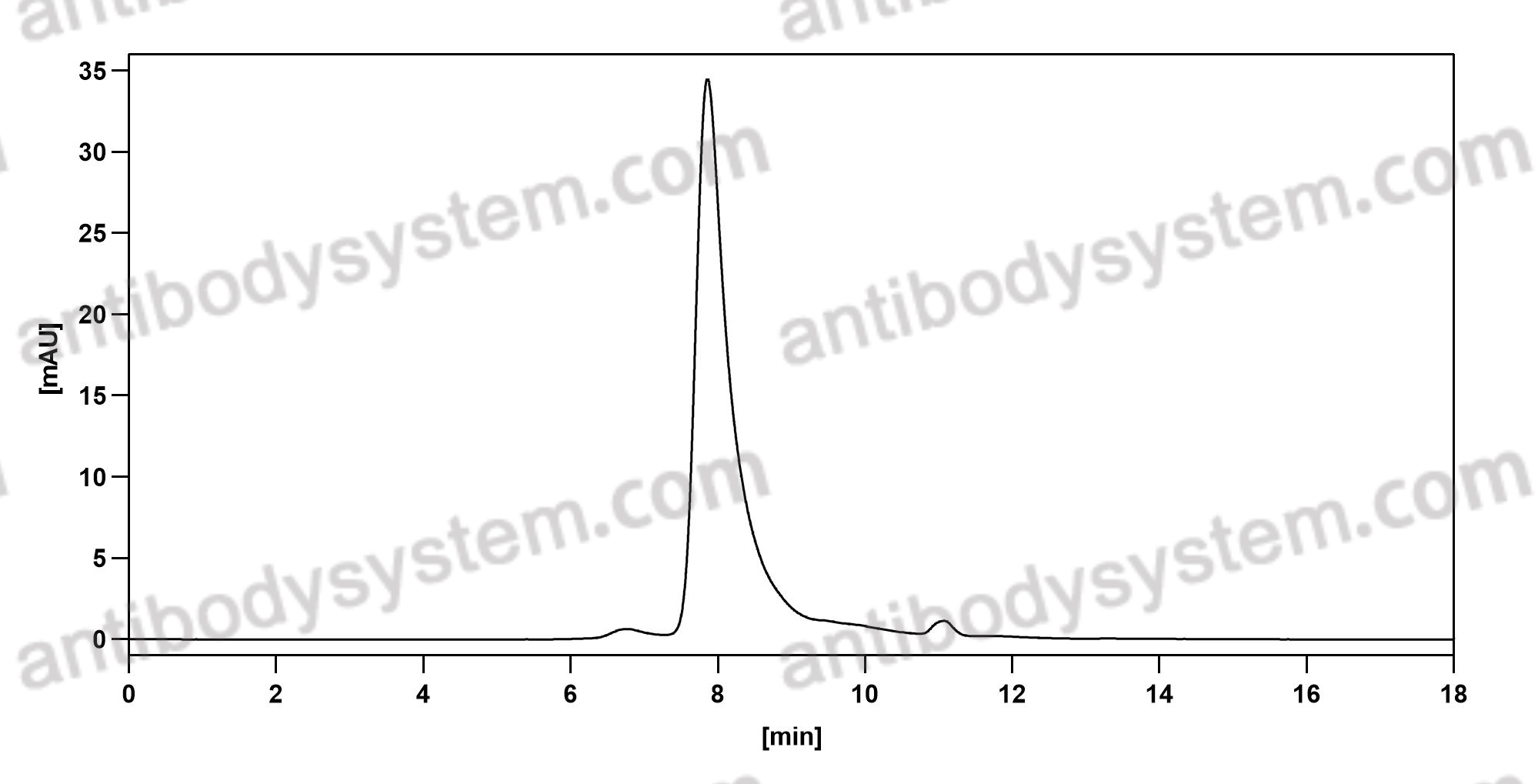
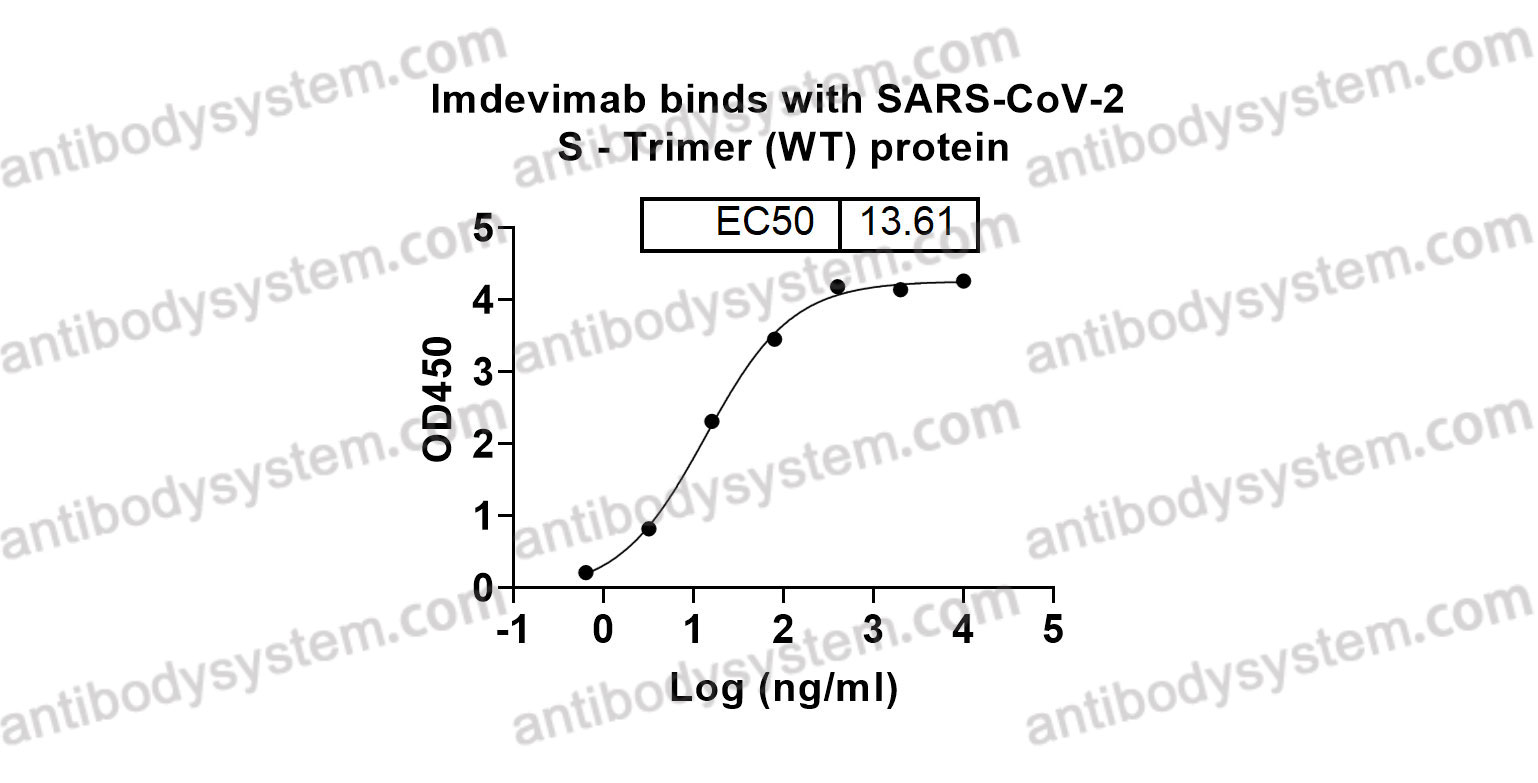
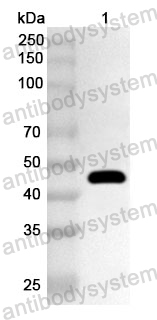
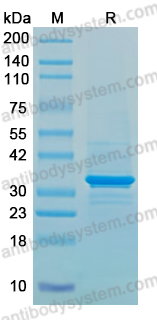
Imdevimab, PMID: 33226741
An EUA for casirivimab and imdevimab for COVID-19, PMID: 33451174
REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, PMID: 33332778
COVID-19 vaccines: The status and perspectives in delivery points of view, PMID: 33359141
Imdevimab, PMID: 34283431
Antibodies to watch in 2021, PMID: 33459118
REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters, PMID: 33037066
Casirivimab, PMID: 33226742
Expanded Access Programs, compassionate drug use, and Emergency Use Authorizations during the COVID-19 pandemic, PMID: 33253920
Initial Guidance on Use of Monoclonal Antibody Therapy for Treatment of Coronavirus Disease 2019 in Children and Adolescents, PMID: 33388760
Casirivimab-imdevimab for Treatment of COVID-19 in Solid Organ Transplant Recipients: An Early Experience, PMID: 33724242
Casirivimab, PMID: 34283490
Emergent Inpatient Administration of Casirivimab and Imdevimab Antibody Cocktail for the Treatment of COVID-19 Pneumonia, PMID: 34194882
Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern, PMID: 34435337
Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab among High-Risk Patients with Mild to Moderate Coronavirus Disease 2019, PMID: 34279629
Neutralizing Antibody Therapeutics for COVID-19, PMID: 33916927
In Translation: FcRn across the Therapeutic Spectrum, PMID: 33802650
Monoclonal antibodies for treating COVID-19, PMID: 33597176
Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19, PMID: 34347950
COVID-19: potential therapeutics for pediatric patients, PMID: 34458951
Real world utilization of REGEN-COV2 at a community hospital, PMID: 34364110
Monoclonal Antibodies Receive EUA to Treat Mild to Moderate COVID-19, PMID: 33497123
Clinical Management of COVID-19: A Review of Pharmacological Treatment Options, PMID: 34071185
Editorial: Post-Exposure Prophylactic Neutralizing Monoclonal Antibodies to SARS-CoV-2 for Individuals at High Risk for COVID-19, PMID: 34393218
The Impact on Infectivity and Neutralization Efficiency of SARS-CoV-2 Lineage B.1.351 Pseudovirus, PMID: 33917138
Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention, PMID: 34159344
Perspectives on passive antibody therapy and peptide-based vaccines against emerging pathogens like SARS-CoV-2, PMID: 34422699
Limited neutralization of authentic SARS-CoV-2 variants carrying E484K in vitro, PMID: 34223909
An EUA for bamlanivimab and etesevimab for COVID-19, PMID: 33830966
An EUA for sotrovimab for treatment of COVID-19, PMID: 34181630
The 11th Trial of a Cardiovascular Clinical Trialist: Coronavirus-2: Part 5, PMID: 33758124
Post Acute Coronavirus (COVID-19) Syndrome, PMID: 34033370
The basis of a more contagious 501Y.V1 variant of SARS-CoV-2, PMID: 33893398
Use of monoclonal antibody therapy for nosocomial SARS-CoV-2 infection in patients at high risk for severe COVID-19: experience from a tertiary-care hospital in Germany, PMID: 34244967
An update to monoclonal antibody as therapeutic option against COVID-19, PMID: 33585808
SARS-CoV-2 Variants: A Synopsis of In Vitro Efficacy Data of Convalescent Plasma, Currently Marketed Vaccines, and Monoclonal Antibodies, PMID: 34201767
Successful Treatment of Persistent Coronavirus Disease 2019 Infection in a Patient With Hypogammaglobulinemia With REGN-COV2: A Case Report, PMID: 34405092
Molecular Perspectives of SARS-CoV-2: Pathology, Immune Evasion, and Therapeutic Interventions, PMID: 34059561
Implementing a method for engineering multivalency to substantially enhance binding of clinical trial anti-SARS-CoV-2 antibodies to wildtype spike and variants of concern proteins, PMID: 34006961
Monoclonal Antibodies: Medical Uses for the Prevention and Treatment of Disease, PMID: 33861166
Neutralizing Monoclonal Antibody Treatment Reduces Hospitalization for Mild and Moderate COVID-19: A Real-World Experience, PMID: 34166513
Subcutaneous REGEN-COV Antibody Combination in Early SARS-CoV-2 Infection, PMID: 34159343
Effectiveness of Severe Acute Respiratory Syndrome Coronavirus 2 Monoclonal Antibody Infusions in High-Risk Outpatients, PMID: 34258319
Prospective mapping of viral mutations that escape antibodies used to treat COVID-19, PMID: 33495308
Interventions in an Ambulatory Setting to Prevent Progression to Severe Disease in Patients With COVID-19: A Systematic Review, PMID: 34157890
A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID-19 in skilled care facilities, PMID: 33619724
The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization, PMID: 34034784
Monoclonal Antibodies for Early Treatment of COVID-19 in a World of Evolving SARS-CoV-2 Mutations and Variants, PMID: 34291118
Treatment of SARS-CoV-2 relapse with remdesivir and neutralizing antibodies cocktail in a patient with X-linked agammaglobulinaemia, PMID: 34332085
Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines, PMID: 33724631
Efficacy and safety of casirivimab/imdevimab (REGEN-COV) in pregnant individuals with COVID-19: Literature review and insights from the COVID-19 International Drug Pregnancy Registry., PMID:40503495
Effectiveness of Anti-SARS-CoV-2 monoclonal antibodies in real-life: RNAemia and clinical outcomes in high-risk COVID-19 patients., PMID:40279347
New Function for Safety Signal Monitoring in MID-NET®: The Case of an Anti-COVID-19 Drug., PMID:40171834
Effectiveness of pharmacological treatments for COVID-19 due to SARS-CoV-2: a systematic literature review., PMID:40093322
Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024., PMID:40084420
Monoclonal Antibodies in Prevention and Early Treatment of COVID-19 in Lung Transplant Recipients: A Systematic Review and Perspective on the Role of Monoclonal Antibodies in the Future., PMID:39995815
Innate and SARS-CoV-2 specific adaptive immune response kinetic in neutralizing monoclonal antibody successfully treated COVID-19 patients., PMID:39832460
Host cell lectins ASGR1 and DC-SIGN jointly with TMEM106B confer ACE2 independence and imdevimab resistance to SARS-CoV-2 pseudovirus with spike mutation E484D., PMID:39791910
Monoclonal antibodies against the spike protein alter the endogenous humoral response to SARS-CoV-2 vaccination and infection., PMID:39504352
Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: A Multicentric Retrospective Study., PMID:39459877
Assessing the safety and pharmacokinetics of casirivimab and imdevimab (CAS+IMD) in a cohort of pregnant outpatients with COVID-19: results from an adaptive, multicentre, randomised, double-blind, phase 1/2/3 study., PMID:39384241
Safety and Pharmacokinetics of Casirivimab and Imdevimab (CAS + IMD) in Pediatric Outpatients With COVID-19., PMID:39351798
Adverse events associated with SARS-CoV-2 neutralizing monoclonal antibodies using the FDA adverse event reporting system database., PMID:39345748
Population Pharmacokinetics of Casirivimab and Imdevimab in Pediatric and Adult Non-Infected Individuals, Pediatric and Adult Ambulatory or Hospitalized Patients or Household Contacts of Patients Infected with SARS-COV-2., PMID:39294447
A Retrospective Cohort Observational Study to Assess the Efficacy of Monoclonal Antibody in Coronavirus Disease 2019 Patients., PMID:39291521
Potential immunomodulatory effects of CAS+IMD monoclonal antibody cocktail in hospitalized patients with COVID-19., PMID:39270622
Monoclonal antibodies against SARS-CoV-2 to prevent COVID-19 worsening in a large multicenter cohort., PMID:39247344
Antiviral therapy for COVID-19 virus: A narrative review and bibliometric analysis., PMID:39244809
Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial., PMID:39236733
Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities., PMID:39126484
Clinical outcomes in patients with mild to moderate coronavirus disease 2019 treated with monoclonal antibody therapy versus an untreated control cohort., PMID:39066463
Sotrovimab in the treatment of coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis of randomized clinical trials., PMID:39031183
Safety and Efficacy of SAB-185 for Nonhospitalized Adults With COVID-19: A Randomized Clinical Trial., PMID:39028902
Ronapreve (REGN-CoV; casirivimab and imdevimab) reduces the viral burden and alters the pulmonary response to the SARS-CoV-2 Delta variant (B.1.617.2) in K18-hACE2 mice using an experimental design reflective of a treatment use case., PMID:39012120
A review on the current approaches and perspectives of Covid-19 treatment., PMID:39007473
Efficacy and safety of casirivimab and imdevimab for preventing and treating COVID-19: a systematic review and meta-analysis., PMID:38983147
Prophylactic antibodies inhibit spike-specific T and B cell responses after COVID-19 vaccination., PMID:38965882
Patient-Reported Outcomes in COVID-19 Treatment with Monoclonal Antibodies Reveal Benefits in Return to Usual Activities., PMID:38961047
Unveiling therapeutic dynamics: An in-depth comparative analysis of neutralizing monoclonal antibodies and favipiravir in alleviating COVID-19 outpatients impacts among middle-aged and special populations (MA-FAST)., PMID:38865775
Successful use of tocilizumab and casirivimab/imdevimab in a twin pregnancy with critical COVID-19 - A case report., PMID:38828309
Casirivimab-imdevimab monoclonal antibody treatment for an immunocompromised patient with persistent SARS-CoV-2 infection: a case report., PMID:38824216
Comparison of bamlanivimab with or without etesevimab and casirivimab-imdevimab in clinical outcomes in patients with COVID-19: a systematic review and meta-analysis., PMID:38721976
Treatment of Patients After Lung Transplantation With Covid Infection During Long-Term Follow-Up., PMID:38714369
Viral Kinetics Model of SARS-CoV-2 Infection Informs Drug Discovery, Clinical Dose, and Regimen Selection., PMID:38676291
Development and validation of a prediction score for failure to casirivimab/imdevimab in hospitalized patients with COVID-19 pneumonia., PMID:38529120
Clinical and Virological Outcome of Monoclonal Antibody Therapies Across SARS-CoV-2 Variants in 245 Immunocompromised Patients: A Multicenter Prospective Cohort Study., PMID:38445721
Effectiveness of subcutaneous monoclonal antibody treatment in emergency department outpatients with COVID-19., PMID:38384380
Relapse of COVID-19 and Viral Evolution in a Patient With Good Syndrome: A Case Report., PMID:38371040
Warm autoimmune hemolytic anemia and hemophagocytic lymphohistiocytosis/macrophage activation syndrome occurring after COVID19 infection and administration of Casirivimab + Imdevimab (COVID19 monoclonal antibody)., PMID:38348150
Coronavirus Disease 2019 (COVID-19) in Heart Transplant Recipients and Anti-SARS-CoV-2 Monoclonal Antibodies: Experience, Lessons Learnt, and Future Challenges., PMID:38334977
Plant-derived compounds as potential leads for new drug development targeting COVID-19., PMID:38281731
Real-world prescription of anti-COVID-19 drugs in hospitalized patients with COVID-19 in Japan., PMID:38277429
Clinical Efficacy of Imdevimab/Casirivimab for Persistent Omicron SARS-CoV-2 Infection in Patients with Hematological Malignancies., PMID:38171874
[Use of a combination of the virus-neutralizing monoclonal antibodies casirivimab and imdevimab for mild to moderate COVID-19 in patients at high risk of progression: Results of the non-interventional observational study]., PMID:38158969
The II Brazilian Guidelines for the pharmacological treatment of patients hospitalized with COVID-19 Joint Guidelines of the Associação Brasileira de Medicina de Emergência, Associação de Medicina Intensiva Brasileira, Associação Médica Brasileira, Sociedade Brasileira de Angiologia e Cirurgia Vascular, Sociedade Brasileira de Infectologia, Sociedade Brasileira de Pneumologia e Tisiologia and Sociedade Brasileira de Reumatologia., PMID:38133154
Nasopharyngeal Viral Load Is the Major Driver of Incident Antibody Immune Response to SARS-CoV-2 Infection., PMID:38111750
Neutralizing monoclonal antibodies for the prevention of severe COVID-19: a retrospective study during Omicron BA.1 variant surge., PMID:38095569
The proteolytic airway environment associated with pneumonia acts as a barrier for treatment with anti-infective antibodies., PMID:38086491
Randomized trial of the safety and efficacy of anti-SARS-CoV-2 mAb in the treatment of patients with nosocomial COVID-19 (CATCO-NOS)., PMID:38058498
Efficacy and safety of casirivimab-imdevimab combination on COVID-19 patients: A systematic review and meta-analysis randomized controlled trial., PMID:38058433