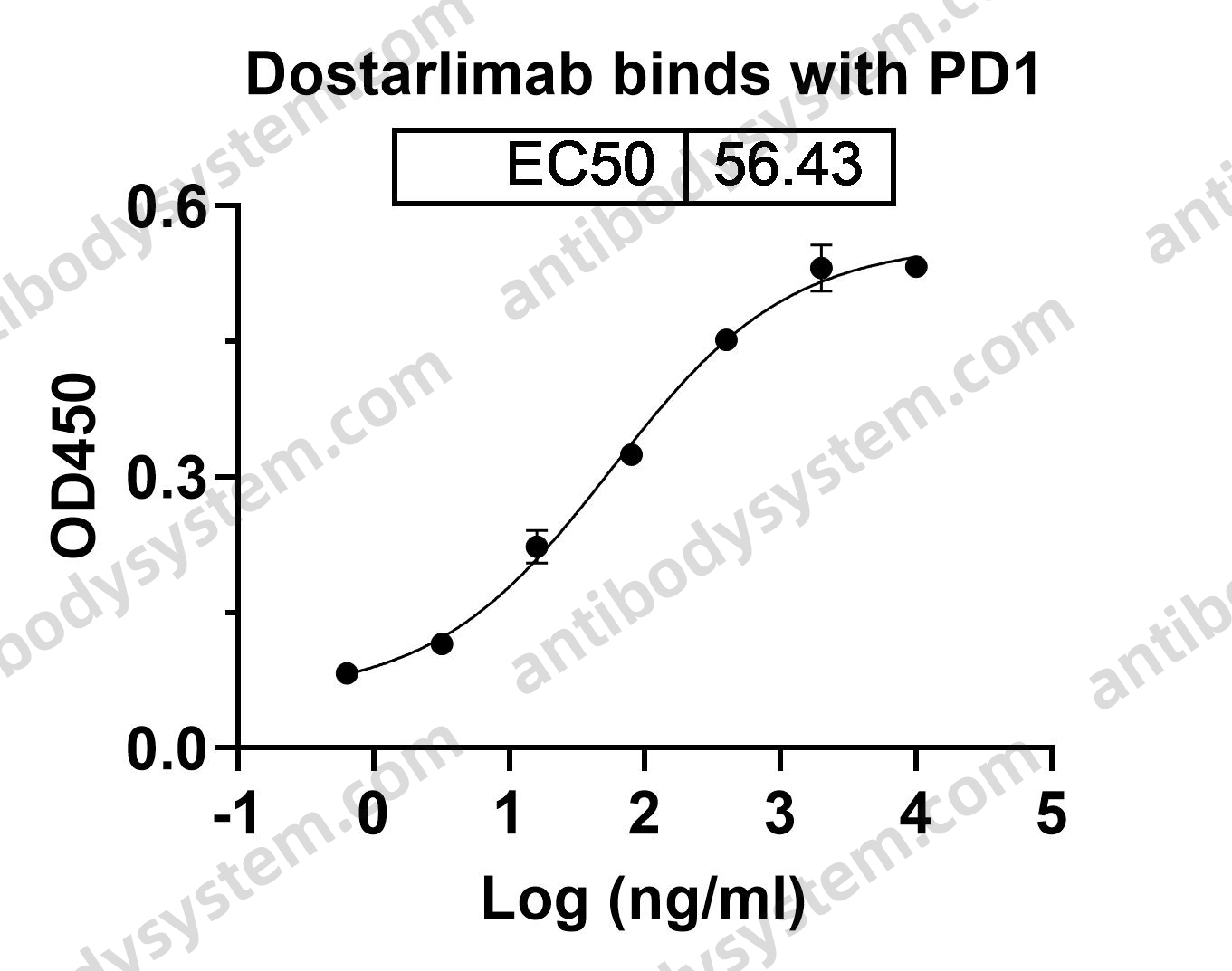
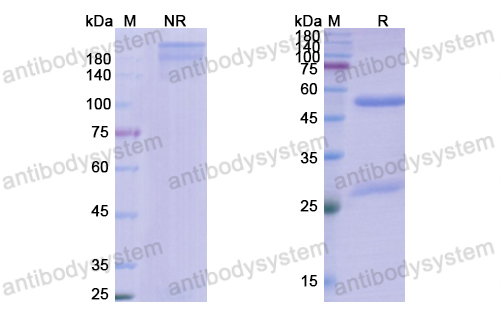
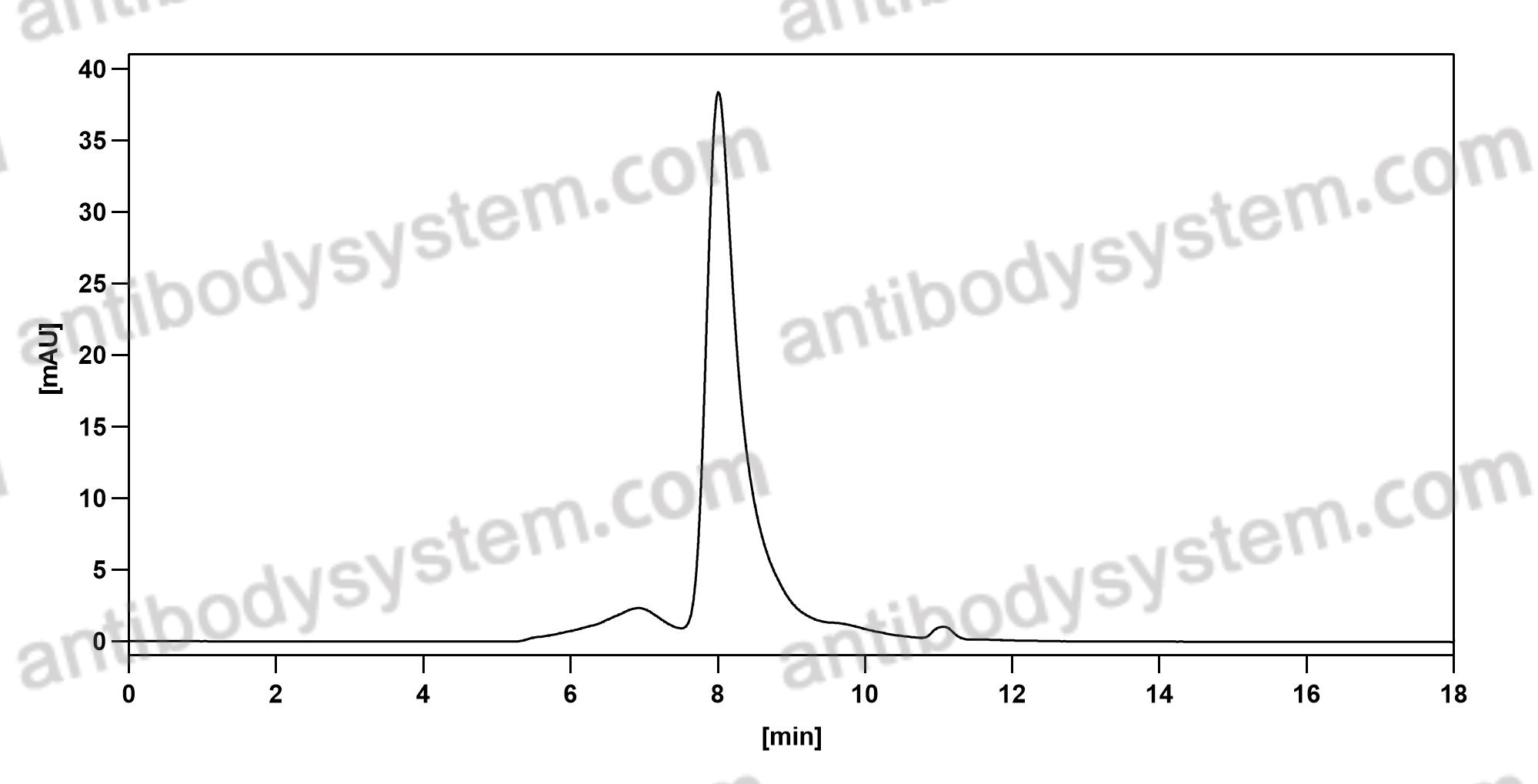
Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial, PMID: 33001143
Dostarlimab in the treatment of recurrent or primary advanced endometrial cancer, PMID: 33251877
Antibodies to watch in 2020, PMID: 31847708
Dostarlimab-gxly, PMID: 34136921
Dostarlimab: First Approval, PMID: 34106455
A Prospective Phase II Single-arm Study of Niraparib Plus Dostarlimab in Patients With Advanced Non-small-cell Lung Cancer and/or Malignant Pleural Mesothelioma, Positive for PD-L1 Expression and Germline or Somatic Mutations in the DNA Repair Genes: Rationale and Study Design, PMID: 32917522
Dostarlimab, PMID: 34014626
A Review of Immune Checkpoint Blockade Therapy in Endometrial Cancer, PMID: 32213091
An Integrated Analysis of Dostarlimab Immunogenicity, PMID: 34324079
Dostarlimab for the treatment of endometrium cancer and other solid tumors, PMID: 33729216
Preclinical characterization of dostarlimab, a therapeutic anti-PD-1 antibody with potent activity to enhance immune function in in vitro cellular assays and in vivo animal models, PMID: 34313545
[New drug approval: Dostarlimab - second line in advanced MSI endometrial cancer], PMID: 33994164
Clinical activity and safety of the anti-PD-1 monoclonal antibody dostarlimab for patients with recurrent or advanced dMMR endometrial cancer, PMID: 34427115
18 F-FDG PET/CT in Relapsed Endometrial Cancer Treated with Preoperative PD-1 Inhibitor Dostarlimab, PMID: 34441288
Endometrial Carcinoma: Immune Microenvironment and Emerging Treatments in Immuno-Oncology, PMID: 34199461
[MSI testing : What is new? What should be considered? German version], PMID: 34043067
Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design, PMID: 33682447
Erratum to 'Phase 2b, open-label, single-arm, multicenter pilot study of the efficacy, safety, and tolerability of dostarlimab in women with early-stage mismatch repair-deficient endometrioid endometrial adenocarcinoma' [International Journal of Gynecological Cancer Volume 35 Issue 4 (2025) 101644]., PMID:40506308
Cost-effectiveness of chemotherapy in advanced and recurrent endometrial cancer., PMID:40487854
Dostarlimab and niraparib in primary advanced ovarian cancer., PMID:40461381
Interview with the World Class Authorities Frontiers of Cancer Research: An exclusive interview with Professor Luis Diaz., PMID:40385346
An ambispective, real-world multi-center study of DOstarlimab in patients with Recurrent or Advanced DNA mismatch repair deficient/microsatellite instability-high endometrial cancer (GEICO 120-R/DORA study)., PMID:40382973
Phase II Study Evaluating the Efficacy of Niraparib and Dostarlimab (TSR-042) in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma., PMID:40377969
[Pembrolizumab or dostarlimab in association to chemotherapy in advanced endometrial cancer]., PMID:40348649
Extended neoadjuvant dostarlimab provides durable benefit in resectable dMMR solid tumours., PMID:40335758
DOMENICA: dostarlimab versus chemotherapy alone in first-line MMR-deficient advanced endometrial cancer patients., PMID:40323277
Rectal stenosis after immunotherapy in a mismatch repair deficient rectal cancer. Case report and review of literature., PMID:40308628
Intratumoral Heterogeneity of Mismatch Repair Status and Outcome of Treatment With Dostarlimab in a Patient With Locally Advanced Rectal Adenocarcinoma., PMID:40294354
Nonoperative Management of Mismatch Repair-Deficient Tumors., PMID:40293177
Immune checkpoint Inhibitor-Induced Autoimmune hemolytic anemia in endometrial cancer., PMID:40292083
Pharmacovigilance study on the reporting frequency of atrial fibrillation with immune checkpoint inhibitors: insights from FDA Adverse Event Reporting System., PMID:40290514
Screening and prevention of gynecologic malignancies in patients with lynch syndrome: following the guidelines., PMID:40144212
A Phase Two, Single-Arm, Open-Label Study With Dostarlimab Monotherapy in Participants With Untreated Stage II/III dMMR/MSI-H Locally Advanced Rectal Cancer (AZUR-1)., PMID:40107952
Molecular Testing and Targeted Therapies in Hepatobiliary Cancers: A Review., PMID:40105823
Releasing the brakes: the role of immune checkpoint inhibitors in laryngeal cancer., PMID:40061133
CITRINO: phase 1 dose escalation study of anti-LAG-3 antibody encelimab alone or in combination with anti-PD-1 dostarlimab in patients with advanced/metastatic solid tumours., PMID:40016550
Phase 2b, open-label, single-arm, multicenter pilot study of the efficacy, safety, and tolerability of dostarlimab in women with early-stage mismatch repair-deficient endometrioid endometrial adenocarcinoma., PMID:39955187
Clinical Use of Dostarlimab in Advanced Stage and Recurrent Endometrial Cancer: Patient Selection and Perspectives., PMID:39881947
Treatment strategies for advanced and recurrent endometrial cancer using immune checkpoint inhibitors., PMID:39812928
Phase II randomized study of dostarlimab alone or with bevacizumab versus non-platinum chemotherapy in recurrent gynecological clear cell carcinoma (DOVE/APGOT-OV7/ENGOT-ov80)., PMID:39710508
Role of PD-1/PD-L1 signaling axis in oncogenesis and its targeting by bioactive natural compounds for cancer immunotherapy., PMID:39690423
If it is a solid tumor target, then it may be a hematologic cancer target: Bridging the great divide., PMID:39689708
The Role of Immunotherapy in MMR-Deficient Endometrial Carcinoma: State of the Art and Future Perspectives., PMID:39685500
Advances in Immunotherapy for Endometrial Cancer: Insights into MMR Status and Tumor Microenvironment., PMID:39682106
Cardiotoxicity associated with immune checkpoint inhibitors: Systematic review and meta-analysis., PMID:39667715
Efficacy and safety of dostarlimab in combination with chemotherapy in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer in a phase 3, randomized, placebo-controlled trial (ENGOT-EN6-NSGO/GOG-3031/RUBY)., PMID:39531903
Cost-effectiveness of dostarlimab plus carboplatin-paclitaxel for primary advanced or recurrent endometrial cancer from a US payer perspective., PMID:39520771
Population pharmacokinetics and exposure-response relationships of dostarlimab in primary advanced or recurrent endometrial cancer in part 1 of RUBY., PMID:39520048
Histology Agnostic Drug Development: An Updated Review., PMID:39518080
Immune Checkpoint Inhibitor Myopathy: The Double-Edged Sword of Cancer Immunotherapy., PMID:39514829
Redefining pancreatic cancer management with tumor-agnostic precision medicine., PMID:39514550
[Practice-changing clinical trials in gastrointestinal radiation oncology]., PMID:39426846
Dostarlimab A new hope for endometrial cancer patients in Pakistan., PMID:39407404
Patient-reported outcomes in patients with metastatic non-squamous non-small cell lung cancer from the randomized Phase II PERLA trial comparing first-line chemotherapy plus dostarlimab or pembrolizumab., PMID:39378565
Curative immune checkpoint inhibitors therapy in patients with mismatch repair-deficient locally advanced rectal cancer: a real-world observational study., PMID:39357124
Safety of dostarlimab in combination with chemotherapy in patients with primary advanced or recurrent endometrial cancer in a phase III, randomized, placebo-controlled trial (ENGOT-EN6-NSGO/GOG-3031/RUBY)., PMID:39346117
Secondary Hemophagocytic Lymphohistiocytosis Following Dostarlimab Treatment in a Patient With Metastatic Endometrial Cancer., PMID:39328329
Patient-reported outcomes in the subpopulation of patients with mismatch repair-deficient/microsatellite instability-high primary advanced or recurrent endometrial cancer treated with dostarlimab plus chemotherapy compared with chemotherapy alone in the ENGOT-EN6-NSGO/GOG3031/RUBY trial., PMID:39322611
Guidelines of Onkopedia: What Is New? Locally Advanced Rectal Cancer., PMID:39288743
Bilateral Panuveitis After Endometrial Cancer Treatment with Dostarlimab: A Case Report., PMID:39284117
Budget impact of dostarlimab plus carboplatin-paclitaxel for primary advanced or recurrent endometrial cancer from a third-party US payer perspective., PMID:39254489
Psoriasis and psoriatic arthritis following use of dostarlimab for endometrial cancer., PMID:39097324
Target-Driven Tissue-Agnostic Drug Approvals-A New Path of Drug Development., PMID:39061168
Dostarlimab: A promising new PD-1 inhibitor for cancer immunotherapy., PMID:39056234
Adverse Events of PD-1, PD-L1, CTLA-4, and LAG-3 Immune Checkpoint Inhibitors: An Analysis of the FDA Adverse Events Database., PMID:39051335
The evolving role of immune checkpoint inhibitors in cervical and endometrial cancer., PMID:39050882
Dostarlimab in the treatment of mismatch repair deficient recurrent or advanced endometrial cancer., PMID:39027143