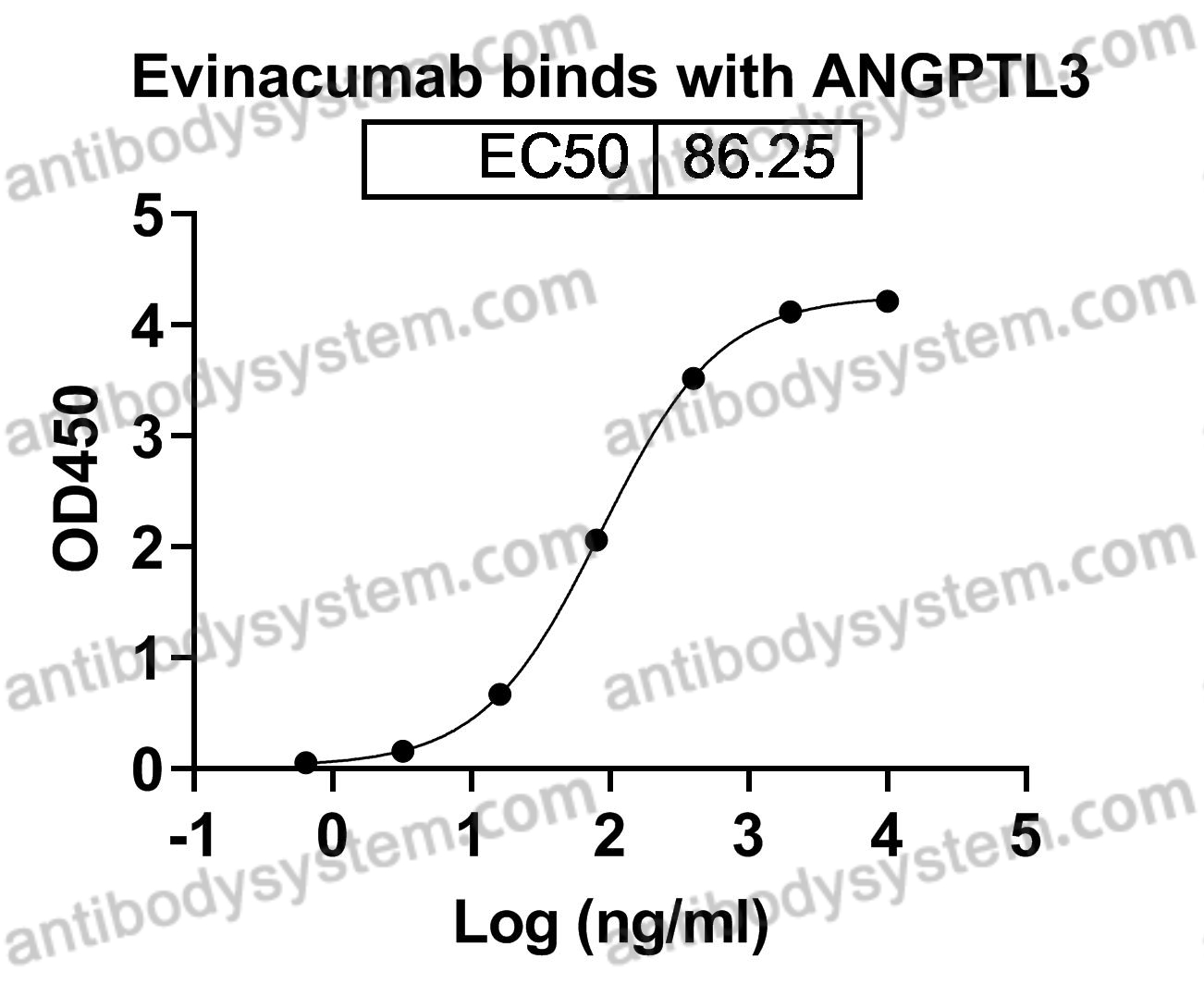
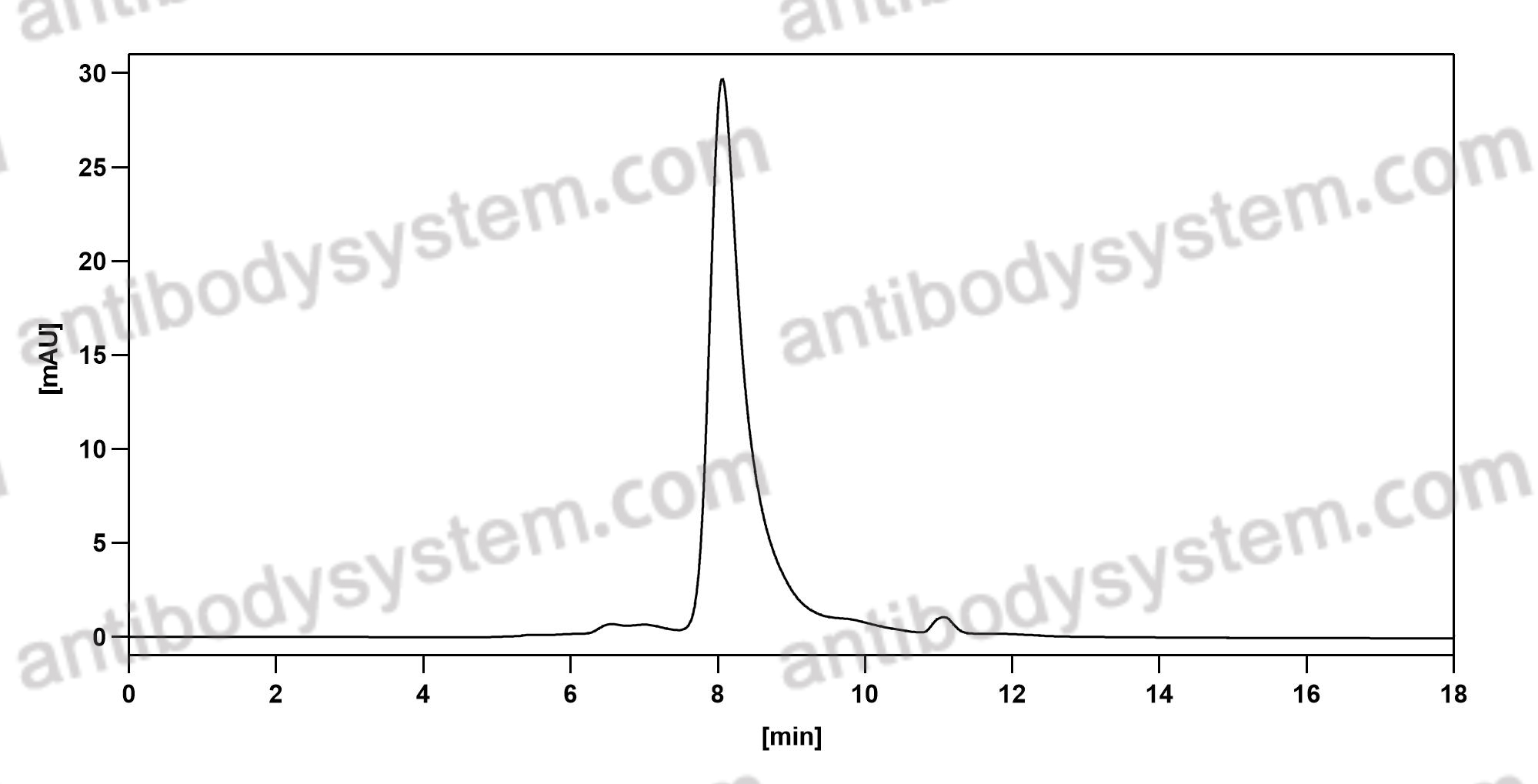
Evinacumab for Homozygous Familial Hypercholesterolemia, PMID: 32813947
Evinacumab in Patients with Refractory Hypercholesterolemia, PMID: 33196153
Functional Analysis of LDLR (Low-Density Lipoprotein Receptor) Variants in Patient Lymphocytes to Assess the Effect of Evinacumab in Homozygous Familial Hypercholesterolemia Patients With a Spectrum of LDLR Activity, PMID: 31578082
Will evinacumab become the standard treatment for homozygous familial hypercholesterolemia?, PMID: 33295805
Antibodies to watch in 2020, PMID: 31847708
Evinacumab, PMID: 34292698
Evinacumab: First Approval, PMID: 34003472
Evinacumab-dgnb, PMID: 33881136
Lipid-Lowering Agents, PMID: 30702996
Inhibition of Angiopoietin-Like Protein 3 With a Monoclonal Antibody Reduces Triglycerides in Hypertriglyceridemia, PMID: 31242752
Alirocumab, evinacumab, and atorvastatin triple therapy regresses plaque lesions and improves lesion composition in mice, PMID: 31843957
ANGPTL3 Inhibition in Homozygous Familial Hypercholesterolemia, PMID: 28723334
Cholesterol Lowering Drugs, PMID: 27809434
A randomized study investigating the safety, tolerability, and pharmacokinetics of evinacumab, an ANGPTL3 inhibitor, in healthy Japanese and Caucasian subjects, PMID: 33130482
Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease, PMID: 28538136
Novel emerging therapies in atherosclerosis targeting lipid metabolism, PMID: 32363959
Evinacumab for treatment of familial hypercholesterolemia, PMID: 34253139
Evinacumab for Homozygous Familial Hypercholesterolemia, PMID: 33567206
Evinacumab for Homozygous Familial Hypercholesterolemia, PMID: 33567205
[Evinacumab in patients with treatment-refractory hypercholesterolemia], PMID: 34014366
Evinacumab for Homozygous Familial Hypercholesterolemia, PMID: 33567207
Evinacumab (Evkeeza) for homozygous familial hypercholesterolemia, PMID: 33976097
Evinacumab for Homozygous Familial Hypercholesterolemia. Reply, PMID: 33567208
Targeting apoC-III and ANGPTL3 in the treatment of hypertriglyceridemia, PMID: 32511037
Lipid lowering therapy in cardiovascular disease: From myth to molecular reality, PMID: 32492513
Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia, PMID: 31931117
The efficacy and safety of evinacumab for the treatment of hypercholesterolemia: a meta-analysis, PMID: 34117182
Angiopoietin-Like 3 (ANGPTL3) and Atherosclerosis: Lipid and Non-Lipid Related Effects, PMID: 30011918
Highlights of Studies in Cardiovascular Disease Prevention Presented at the 2020 American College of Cardiology Annual Scientific Session, PMID: 32556778
Volanesorsen in the Treatment of Familial Chylomicronemia Syndrome or Hypertriglyceridaemia: Design, Development and Place in Therapy, PMID: 32753844
[Familial chylomicronemia], PMID: 32841138
Factoring in ANGPTL3 When LDL Is Refractory, PMID: 33296565
Evinacumab Approval Adds a New Option for Homozygous Familial Hypercholesterolemia With a Hefty Price Tag, PMID: 34152800
Bypassing the LDL Receptor in Familial Hypercholesterolemia, PMID: 32813955
A quantitative systems pharmacology modeling platform for evaluating triglyceride profiles in patients with high triglycerides receiving evinacumab, PMID: 34327869
ANGPTL3 Inhibition With Evinacumab Results in Faster Clearance of IDL and LDL apoB in Patients With Homozygous Familial Hypercholesterolemia-Brief Report, PMID: 33691480
Emerging lipid lowering agents targeting LDL cholesterol, PMID: 32243228
Inhibition of Angiopoietin-Like Protein 3 With Evinacumab in Subjects With High and Severe Hypertriglyceridemia, PMID: 34238441
Advances with lipid-lowering drugs for pediatric patients with familial hypercholesterolemia, PMID: 33016816
ANGPTL3 inhibition lowers LDL-C levels in FH, PMID: 32855535
Angiopoietin-Like 3 Protein Inhibition: A New Frontier in Lipid-Lowering Treatment, PMID: 31008773
Homozygous familial hypercholesterolemia: what treatments are on the horizon?, PMID: 32332430
Biotechnology Approaches for the Treatment of Dyslipidemia, PMID: 32519066
Safety and tolerability of injectable lipid-lowering drugs: an update of clinical data, PMID: 31100030
Novel therapies for familial hypercholesterolemia, PMID: 33278127
Evidence for improved survival with treatment of homozygous familial hypercholesterolemia, PMID: 32520777
Angiopoietin-like proteins as therapeutic targets for cardiovascular disease: focus on lipid disorders, PMID: 31856617
Triglyceride-Rich Lipoproteins and Novel Targets for Anti-atherosclerotic Therapy, PMID: 30403015
Marked plaque regression in homozygous familial hypercholesterolemia, PMID: 34004483
Angiopoietin-like proteins inhibitors: New horizons in the treatment of atherogenic dyslipidemia and familial hypercholesterolemia, PMID: 33470417
Long-term experience with lomitapide treatment in patients with homozygous familial hypercholesterolemia: Over 10 years of efficacy and safety data., PMID:40494715
Global disparities in access to lipid-lowering therapies for patients with homozygous familial hypercholesterolemia - A physician survey., PMID:40480942
ANGPTL3: A Breakthrough Target in Treatment for Dyslipidemia and Atherosclerosis., PMID:40467521
Real-World Effectiveness and Safety of Evinacumab in Children and Adults With Homozygous Familial Hypercholesterolemia: A Multisite US Perspective., PMID:40438928
Evinacumab and reduced lipoprotein apheresis in pediatric homozygous familial hypercholesterolemia: a retrospective study on LDL-C., PMID:40393255
Safety and effectiveness of evinacumab in an infant with homozygous familial hypercholesterolemia: A new renaissance for the very young?, PMID:40187919
Evinacumab for Homozygous Familial Hypercholesterolemia: The Italian Cohort of the ELIPSE HoFH Study., PMID:40169529
Beyond LDL: Targeting ANGPTL3 to Reduce Residual Cardiovascular Risk., PMID:40167412
A Case with Oligogenic Familial Hypercholesterolemia Treated by Combination Therapy of Statin, Ezetimibe, PCSK9 Inhibitor, and Lomitapide., PMID:40128994
Comparison of Model-Predicted and Observed Evinacumab Pharmacokinetics and Efficacy in Children Aged < 5 Years With Homozygous Familial Hypercholesterolemia., PMID:40095766
Population Pharmacokinetics and Exposure-Response Modeling for Evinacumab in Children, Adolescents, and Adults With Homozygous Familial Hypercholesterolemia., PMID:40095399
Evinacumab for the treatment of homozygous familial hypercholesterolaemia: first patient case report in Switzerland., PMID:40062884
Treatment With Evinacumab Links a New Pathogenic Variant in the LPL Gene to Persistent Chylomicronemia., PMID:40046103
Effects of Inclisiran, Alirocumab, Evolocumab, and Evinacumab on Lipids: A Network Meta-Analysis., PMID:40026525
Dramatic response to Evinacumab in a North Indian girl with homozygous familial hypercholesterolemia., PMID:40022592
High burden of disease in patients with homozygous familial hypercholesterolemia despite recent advances in therapies and updated guidelines: A real-world study., PMID:39971631
Evinacumab as an adjunct to lipid apheresis in an infant with homozygous familial hypercholesterolemia., PMID:39970166
Emergency Management of Intoxication-Type Inherited Metabolic Disorders., PMID:39953653
Evinacumab for children with homozygous familial hypercholesterolemia: a plain language summary., PMID:39931838
Efficacy of a novel PCSK9 inhibitory peptide alone and with evinacumab in a mouse model of atherosclerosis., PMID:39909173
Molecular Therapeutics in Development to Treat Hyperlipoproteinemia., PMID:39875700
Genetically mimicked effects of evinacumab on psoriasis: a drug target Mendelian randomization study., PMID:39828257
Exploring emerging pharmacotherapies for type 2 diabetes patients with hypertriglyceridemia., PMID:39794291
Homozygous Familial Hypercholesterolemia Treatment: New Developments., PMID:39751968
Homozygous familial hypercholesterolemia in Spain. Data from Registry of the Spanish Atherosclerosis Society., PMID:39514762
Correction to: Evinacumab in homozygous familial hypercholesterolaemia: long-term safety and efficacy., PMID:39302808
Expanding therapeutic options: overview of novel pharmacotherapies for dyslipidemia., PMID:39286934
Drug interactions in cardiology: focus on statins and their combination with other lipid-lowering drugs., PMID:39252198
Advances in targeting LDL cholesterol: PCSK9 inhibitors and beyond., PMID:39070027
Real-world experience of long-term efficacy and safety of evinacumab in patients with homozygous familial hypercholesterolemia treated and untreated with lipoprotein apheresis., PMID:39054196
Circumventing Cardiovascular Calamities: The Dawn of ANGPTL3 Blockade in Severe Dyslipidemia Management., PMID:39039670
Effects of Lipoproteins on Metabolic Health., PMID:38999903
Repurposing lipid-lowering drugs on asthma and lung function: evidence from a genetic association analysis., PMID:38961500
The Long-Term Efficacy and Safety of Evinacumab in Patients With Homozygous Familial Hypercholesterolemia., PMID:38938723
Evinacumab Therapy for Homozygous Familial Hypercholesterolemia: Driving Lipoprotein Clearance Via the Road Less Taken., PMID:38938707
New Insights Into the Treatment of Hyperlipidemia: Pharmacological Updates and Emerging Treatments., PMID:38919858
Decreased LDL-Cholesterol Exposure Following ANGPTL3 Inhibition Reduces Coronary Plaque Development in Homozygous Familial Hypercholesterolemia., PMID:38864785
Evinacumab in homozygous familial hypercholesterolaemia: long-term safety and efficacy., PMID:38856678
ANGPTL3 as a therapeutic target for treating homozygous familial hypercholesterolaemia: a shot in the arm for evinacumab., PMID:38856677
Evinacumab: Mechanism of action, clinical, and translational science., PMID:38845393
Dyslipidaemia management in pregnant patients: a 2024 update., PMID:38784103
Intensive Combination LDL-Lowering Therapy in a Patient With Homozygous Familial Hypercholesterolemia., PMID:38774638
Safety of cardiovascular disease drugs approved between 2014 and 2021 in the US: a pharmacovigilance analysis., PMID:38722712
Evaluating the Effectiveness and Safety of Evinacumab in Treating Hypercholesterolemia and Hypertriglyceridemia: A Systematic Review and Meta-analysis of Randomized Controlled Trials., PMID:38713309
Evinacumab and Cardiovascular Outcome in Patients With Homozygous Familial Hypercholesterolemia., PMID:38695169
How can we improve the prognosis of patients with homozygous familial hypercholesterolemia?, PMID:38658251
Current treatments for the management of homozygous familial hypercholesterolaemia: a systematic review and commentary., PMID:38640433
A machine-learning algorithm using claims data to identify patients with homozygous familial hypercholesterolemia., PMID:38632285