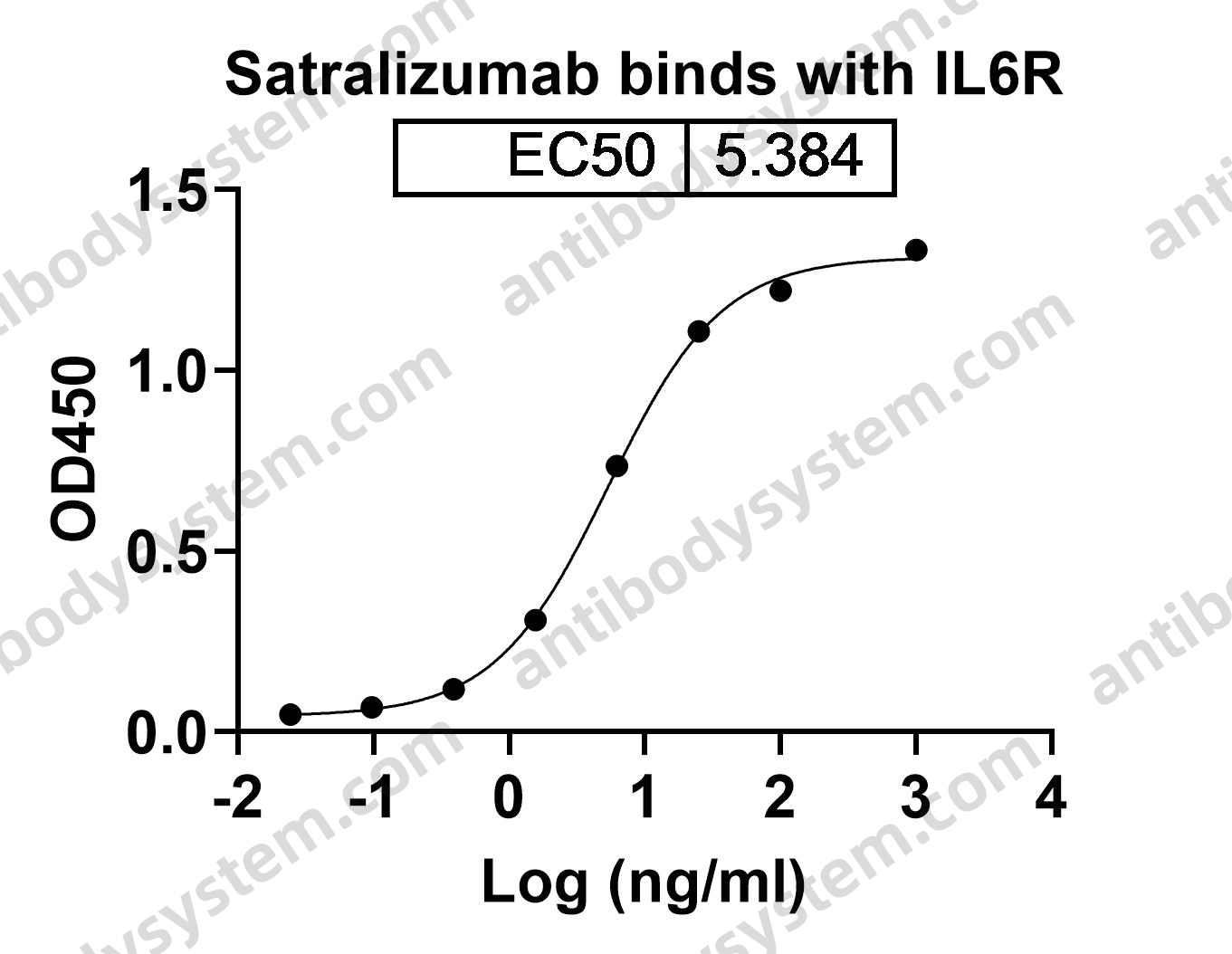
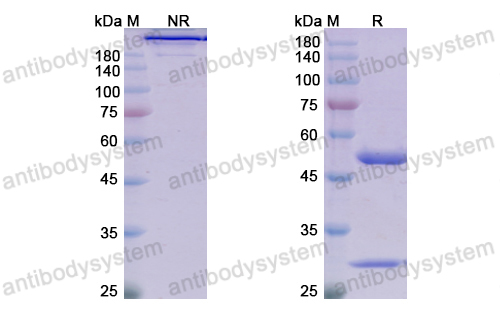
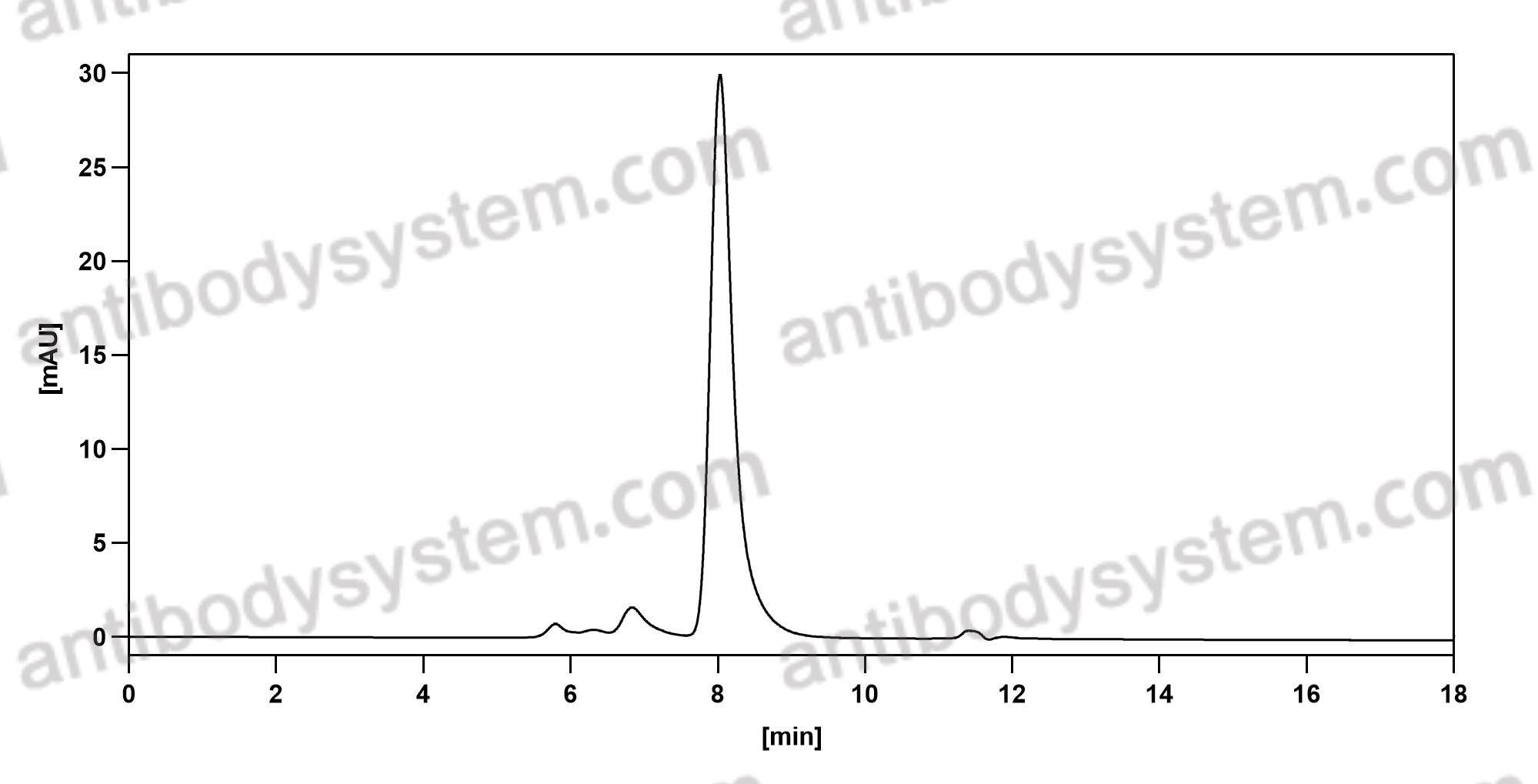
Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder, PMID: 31774956
Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial, PMID: 32333898
Satralizumab: First Approval, PMID: 32797372
Satralizumab-mwge, PMID: 33091105
Satralizumab, PMID: 33355730
Antibodies to watch in 2020, PMID: 31847708
Satralizumab for the Treatment of Neuromyelitis Optica Spectrum Disorders, PMID: 33246373
Satralizumab in the treatment of neuromyelitis optica spectrum disorder, PMID: 33167776
Correction to: Satralizumab: First Approval, PMID: 32902801
Advances in the Treatment of Neuromyelitis Optica Spectrum Disorder, PMID: 33223088
New therapies for neuromyelitis optica spectrum disorder, PMID: 33186537
Review of approved NMO therapies based on mechanism of action, efficacy and long-term effects, PMID: 33059216
Antibodies to watch in 2019, PMID: 30516432
Update on neuromyelitis optica spectrum disorder, PMID: 33009077
Recent progress in maintenance treatment of neuromyelitis optica spectrum disorder, PMID: 33011853
Emerging drugs for the treatment of neuromyelitis optica, PMID: 32731771
Pharmacotherapy for Neuromyelitis Optica Spectrum Disorders: Current Management and Future Options, PMID: 30623348
Novel insights into pathophysiology and therapeutic possibilities reveal further differences between AQP4-IgG- and MOG-IgG-associated diseases, PMID: 32304439
Anti-IL-6 Therapies for Neuromyelitis Optica Spectrum Disorders: A Systematic Review of Safety and Efficacy, PMID: 32348222
Satralizumab: an interleukin-6 (IL-6) receptor antagonist for the treatment of neuromyelitis optica spectrum disorders, PMID: 33729218
Emerging Targeted Therapies for Neuromyelitis Optica Spectrum Disorders, PMID: 33301078
Current and emerging biologics for the treatment of neuromyelitis optica spectrum disorders, PMID: 32228250
Targeting IL-6 receptor in the treatment of neuromyelitis optica spectrum: a review of emerging treatment options, PMID: 32306778
Lupus and NMOSD: The Blending of Humoral Autoimmunity, PMID: 33123402
Efficacy and Safety of Monoclonal Antibody Therapy in Neuromyelitis Optica Spectrum Disorders: Evidence from Randomized Controlled Trials, PMID: 32442886
Immune reconstitution therapy in NMOSD, PMID: 33992916
New Therapeutic Landscape in Neuromyelitis Optica, PMID: 33814893
Antibodies to watch in 2018, PMID: 29300693
[Neuromyelitis Optica Spectrum Disorder], PMID: 34006678
Different Targets of Monoclonal Antibodies in Neuromyelitis Optica Spectrum Disorders: A Meta-Analysis Evidenced From Randomized Controlled Trials, PMID: 33391166
Recent advances in the treatment of neuromyelitis optica spectrum disorders, PMID: 33741809
Drug Treatment of Neuromyelitis Optica Spectrum Disorders: Out with the Old, in with the New?, PMID: 33777853
B Cells and Antibodies as Targets of Therapeutic Intervention in Neuromyelitis Optica Spectrum Disorders, PMID: 33419217
Targeting interleukin-6 to treat neuromyelitis optica spectrum disorders: Implications from immunology, the FcRn pathway and clinical experience, PMID: 33781948
Rituximab for the Treatment of Neuromyelitis Optica Spectrum Disorder [Internet], PMID: 34165925
Targeting Neuromyelitis Optica Pathogenesis: Results from Randomized Controlled Trials of Biologics, PMID: 33909234
[Treatment of antibody-mediated encephalomyelitis : Strategies for the treatment of neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein antibody-associated disease], PMID: 33783551
Effectiveness of treatments in Neuromyelitis optica to modify the course of disease in adult patients. Systematic review of literature, PMID: 33711580
Monoclonal Antibody Therapy in Neuromyelitis Optica Spectrum Disorders: a Meta-analysis of Randomized Control Trials, PMID: 34354581
[A case of neuromyelitis optica associated with pulmonary Mycobacterium avium complex disease], PMID: 34433747
Emerging Role of Targeted Monoclonal Antibodies in Neuromyelitis Optica Spectrum Disorders., PMID:40506618
From checkboxes to emojis: a novel approach to patient-reported outcomes in multiple sclerosis., PMID:40447786
Anti-IL-6R antibody treatment changes microglial phenotype in AQP4 peptide-immunized mice, leading to suppression of myelitis severity., PMID:40403514
Interleukin-6 in neuroimmunological disorders: Pathophysiology and therapeutic advances with satralizumab., PMID:40324548
Clinical Features and Factors Associated With Outcome in Late Adult-Onset Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease., PMID:40324120
Potential drug targets for Neuromyelitis optica spectrum disorders (NMOSD): A Mendelian randomization analysis., PMID:40294019
Assessing fall risk in multiple sclerosis using patient-reported outcomes and wearable gait metrics., PMID:40292035
Analysis of infection rates in neuromyelitis optica spectrum disorder: Comparing satralizumab treatment in SAkuraMoon, post-marketing, and US-based health claims data., PMID:40288333
Very-late-onset neuromyelitis optica spectrum disorder: a case report and review., PMID:40276466
Neuromyelitis optica spectrum disorder in Latin America: a global data share initiative., PMID:40267692
Late Relapse After Autologous Hematopoietic Stem Cell Transplantation in AQP4-IgG-Positive NMOSD., PMID:40257801
Effectiveness of satralizumab in a real-world clinical setting in Japan: Interleukin-6 receptor inhibition in neuromyelitis optica spectrum disorder: A six-month interim analysis of a multicenter medical chart review., PMID:40203604
Satralizumab after inebilizumab treatment in a patient with recurrent neuromyelitis optica spectrum disorder: A case report., PMID:40193676
Ventral Subpial and Central Cord Enhancement in Spinal Neurosarcoidosis: The Reverse Trident Sign., PMID:40168633
Satralizumab treatment in patients with AQP4-IgG-seropositive neuromyelitis optica spectrum disorder after rituximab treatment: A case series., PMID:40132364
Evaluating Rituximab Failure Rates in Neuromyelitis Optica Spectrum Disorder: A Nationwide Real-World Study From South Korea., PMID:40065454
Assessment of concurrent neoplasms and a paraneoplastic association in MOGAD., PMID:39932929
Severe Relapse After Switching From Eculizumab to Satralizumab in Neuromyelitis Optica Spectrum Disorder., PMID:39913883
Real-world clinical experience with serum MOG and AQP4 antibody testing by live versus fixed cell-based assay., PMID:39901660
CSF cytokine, chemokine and injury biomarker profile of glial fibrillary acidic protein (GFAP) autoimmunity., PMID:39869499
Switch from eculizumab to satralizumab in aquaporin 4 immunoglobulin G-seropositive neuromyelitis optica spectrum disorder: a case series report., PMID:39867894
Safety and efficacy of satralizumab in patients with generalised myasthenia gravis (LUMINESCE): a randomised, double-blind, multicentre, placebo-controlled phase 3 trial., PMID:39862880
Testing for myelin oligodendrocyte glycoprotein antibodies: Who, what, where, when, why, and how., PMID:39861933
Curriculum Innovations: A Novel Neurology Clinician-Educator Program., PMID:39748890
[Subjective sleep quality evaluation in patients with neuromyelitis optica spectrum disorders]., PMID:39731371
Autoimmune brainstem encephalitis: Clinical associations, outcomes, and proposed diagnostic criteria., PMID:39708293
Case report: Satralizumab as an adjunctive therapy for AQP-4 antibody and MOG antibody dual-negative optic neuritis in a third-trimester pregnancy case., PMID:39687907
Case report: Satralizumab therapy for bilateral refractory optic neuritis following the first dose of bivalent human papilloma virus vaccine., PMID:39628490
Usage status of biologics for the chronic treatment of optic neuritis in neuromyelitis optica spectrum disorders in Japan., PMID:39470886
An evaluation of ravulizumab for the treatment of neuromyelitis optica spectrum disorder., PMID:39460545
Pediatric MOG-Ab-Associated Encephalitis: Supporting Early Recognition and Treatment., PMID:39393046
Immunogenicity dynamics and covariate effects after satralizumab administration predicted with a hidden Markov model., PMID:39380259
Comprehensive Analysis of Paraneoplastic Neurologic Syndrome and PNS-CARE Diagnostic Criteria in Clinical Practice., PMID:39321395
Intracranial Pressure Elevation and MOGAD., PMID:39283648
Long-term outcomes in antibody-negative autoimmune encephalitis: a retrospective study., PMID:39278895
Blood neurofilament light chain measurements in adults with CNS histiocytic neoplasms., PMID:39237493
Innovation and optimization in autoimmune encephalitis trials: the design and rationale for the Phase 3, randomized study of satralizumab in patients with NMDAR-IgG-antibody-positive or LGI1-IgG-antibody-positive autoimmune encephalitis (CIELO)., PMID:39193150
Visual improvement in a case of neuromyelitis optica spectrum disorder-related optic neuritis after 18 months of treatment with satralizumab: A case report., PMID:39157378
Dysthyroid Optic Neuropathy Complicated by Neuromyelitis Optica Spectrum Disorder: A Case Report., PMID:39144641
The pharmacological management of myelin oligodendrocyte glycoprotein-immunoglobulin G associated disease (MOGAD): an update of the literature., PMID:39110029
Application of the international criteria for optic neuritis in the Acute Optic Neuritis Network., PMID:39099240
Cerebrospinal Fluid Cytokine and Chemokine Profiles in Central Nervous System Sarcoidosis: Diagnostic and Immunopathologic Insights., PMID:39031103
Safety and Effectiveness of Satralizumab in Japanese Patients with Neuromyelitis Optica Spectrum Disorder: A 6-month Interim Analysis of Post-marketing Surveillance., PMID:39012406
Frequency of Asymptomatic Optic Nerve Enhancement in 203 Patients With MOG Antibody-Associated Disease., PMID:38924706
Interleukin-6 Signaling Blockade Induces Regulatory Plasmablasts in Neuromyelitis Optica Spectrum Disorder., PMID:38889374
Network Meta-analysis of Ravulizumab and Alternative Interventions for the Treatment of Neuromyelitis Optica Spectrum Disorder., PMID:38722571
MOG Antibody-Associated Disease in the Setting of Metastatic Melanoma Complicated by Immune Checkpoint Inhibitor Use., PMID:38696737
[Novel immunomodulatory therapies in myasthenia gravis]., PMID:38665106
Monoclonal antibody therapies for aquaporin-4-immunoglobulin G-positive neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein antibody-associated disease., PMID:38628414