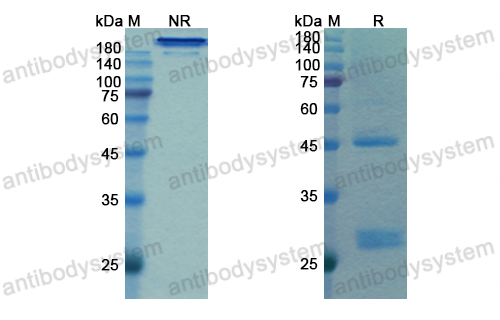
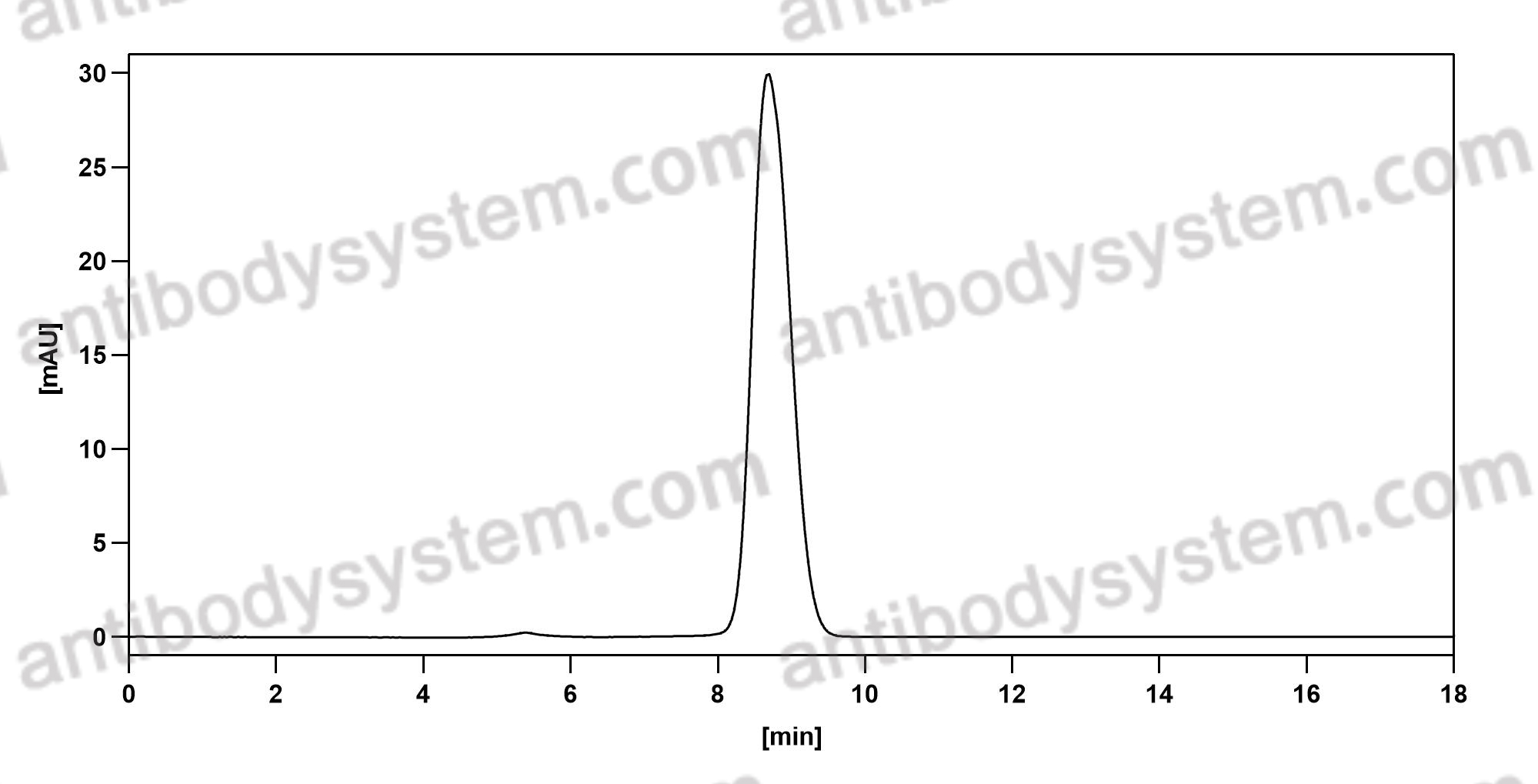
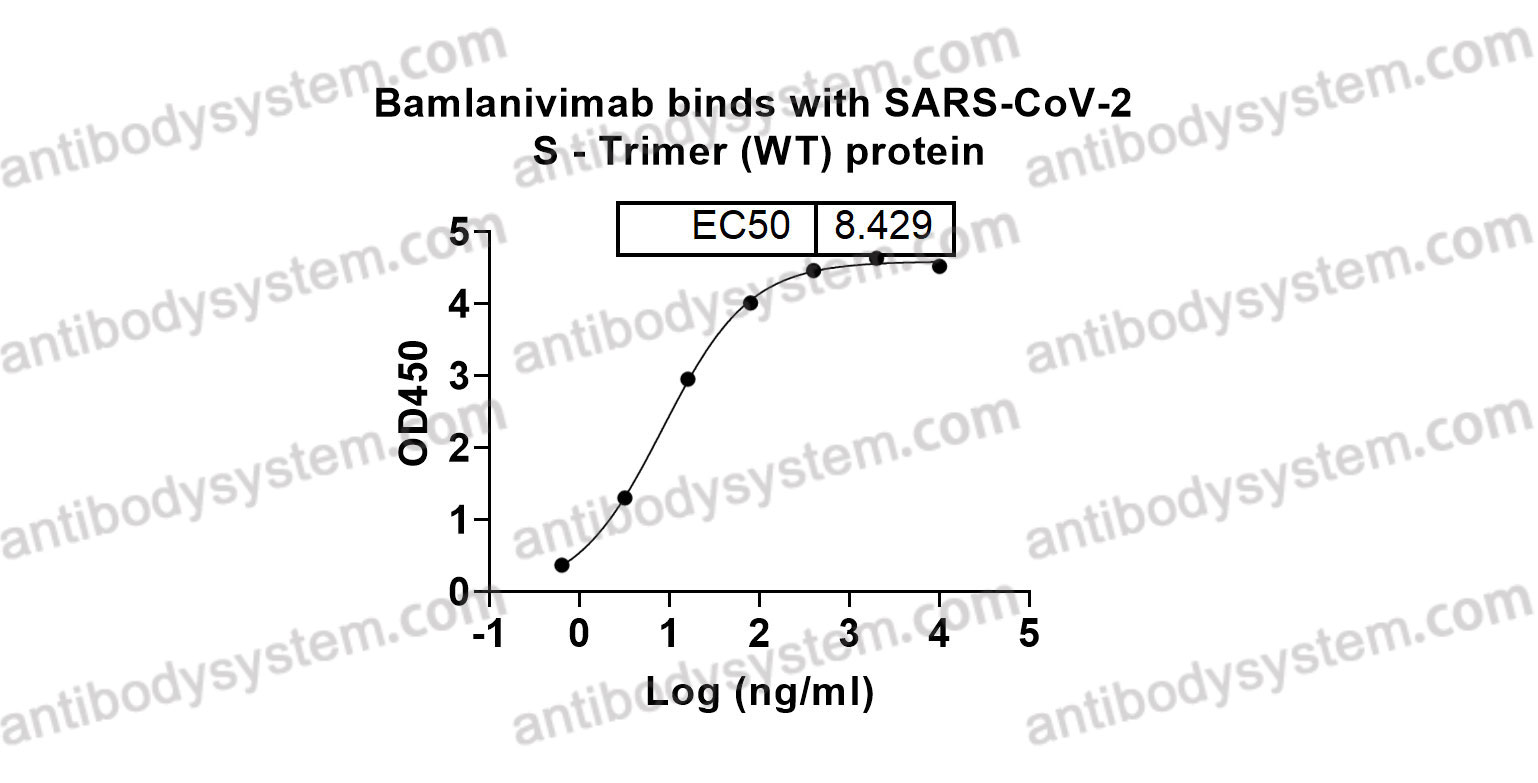
Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, PMID: 33475701
Bamlanivimab, PMID: 33226744
An EUA for Bamlanivimab-A Monoclonal Antibody for COVID-19, PMID: 33306087
Covid-19: FDA authorises neutralising antibody bamlanivimab for non-admitted patients, PMID: 33177042
SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, PMID: 33113295
Antimicrobial stewardship and bamlanivimab: Opportunities for outpatient preauthorization?, PMID: 33213544
COVID-19 vaccines: The status and perspectives in delivery points of view, PMID: 33359141
Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline, PMID: 33319649
Antibodies to watch in 2021, PMID: 33459118
An EUA for bamlanivimab - a monoclonal antibody for COVID-19, PMID: 33443490
A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19, PMID: 33356051
Bamlanivimab for Prevention of COVID-19, PMID: 34081075
Bamlanivimab Use in a Military Treatment Facility, PMID: 33993281
Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, PMID: 34260849
Expanded Access Programs, compassionate drug use, and Emergency Use Authorizations during the COVID-19 pandemic, PMID: 33253920
Efficacy of Bamlanivimab in Reducing Hospitalization and Mortality Rates in COVID-19 Patients in a Rural Community, PMID: 34430093
Etesevimab and Bamlanivimab, PMID: 33630482
Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial, PMID: 34081073
A Narrative Review of the Clinical Practicalities of Bamlanivimab and Etesevimab Antibody Therapies for SARS-CoV-2, PMID: 34374951
Real-World Experience of Bamlanivimab for COVID-19: A Case-Control Study, PMID: 33846730
Initial Guidance on Use of Monoclonal Antibody Therapy for Treatment of Coronavirus Disease 2019 in Children and Adolescents, PMID: 33388760
Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19, PMID: 34411003
An EUA for bamlanivimab and etesevimab for COVID-19, PMID: 33830966
Bamlanivimab as monotherapy in two immunocompromised patients with COVID-19, PMID: 34341781
Impact of Bamlanivimab Monoclonal Antibody Treatment on Hospitalization and Mortality Among Nonhospitalized Adults With Severe Acute Respiratory Syndrome Coronavirus 2 Infection, PMID: 34250192
Bamlanivimab For Mild to Moderate COVID-19 in Kidney Transplant Recipients, PMID: 34222731
Association of Intravenous Bamlanivimab Use with Reduced Hospitalization, Intensive Care Unit Admission, and Mortality in Patients with Mild to Moderate COVID-19, PMID: 34075387
Bamlanivimab infusion experience at one academic emergency department, PMID: 34294501
Bamlanivimab use in mild-to-moderate COVID-19 disease: A matched cohort design, PMID: 34328670
Diffuse C4d staining of peritubular capillaries in renal allograft following bamlanivimab therapy, PMID: 34358400
Etesevimab, PMID: 34283469
First in Human Study of Bamlanivimab in a Randomized Trial of Hospitalized Patients with COVID-19, PMID: 34455583
Initial experience of bamlanivimab monotherapy use in solid organ transplant recipients, PMID: 34081820
Bamlanivimab for treatment of COVID-19 in solid organ transplant recipients: Early single-center experience, PMID: 33595145
Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern, PMID: 34435337
Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany, PMID: 34278371
Bamlanivimab treatment leads to rapid selection of immune escape variant carrying E484K mutation in a B.1.1.7 infected and immunosuppressed patient, PMID: 34009286
The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting, PMID: 34258324
Consequences of rush to emergency use authorization of bamlanivimab, PMID: 34216205
501Y.V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to Bamlanivimab in vitro, PMID: 33619479
Emergency Use Authorization for Bamlanivimab in Mild to Moderate COVID-19: Implications for CNS Practice, PMID: 33793179
501Y.V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to bamlanivimab in vitro, PMID: 34074219
Administration of Bamlanivimab to Skilled Nursing Facility Residents During a COVID-19 Outbreak, January-February 2021, Arizona, PMID: 34000267
Emergency Use Authorization for Bamlanivimab in Mild to Moderate COVID-19: Implications for Clinical Nurse Specialist Practice, PMID: 33793172
Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab among High-Risk Patients with Mild to Moderate Coronavirus Disease 2019, PMID: 34279629
An overview of the preclinical discovery and development of bamlanivimab for the treatment of novel coronavirus infection (COVID-19): reasons for limited clinical use and lessons for the future, PMID: 34304682
Emergence of E484K Mutation Following Bamlanivimab Monotherapy among High-Risk Patients Infected with the Alpha Variant of SARS-CoV-2, PMID: 34452507
Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study, PMID: 33981518
Emergence of Q493R mutation in SARS-CoV-2 spike protein during bamlanivimab/etesevimab treatment and resistance to viral clearance, PMID: 34437928
SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination, PMID: 34270919
The pan-variant potential of light: 425 nm light inactivates SARS-CoV-2 variants of concern and non-cytotoxic doses reduce viral titers in human airway epithelial cells., PMID:40434113
Effectiveness of Anti-SARS-CoV-2 monoclonal antibodies in real-life: RNAemia and clinical outcomes in high-risk COVID-19 patients., PMID:40279347
Regional comparison of efficacy and safety for vilobelimab in critically ill, invasively mechanically ventilated COVID-19 patients., PMID:40250846
Monoclonal Antibodies in Prevention and Early Treatment of COVID-19 in Lung Transplant Recipients: A Systematic Review and Perspective on the Role of Monoclonal Antibodies in the Future., PMID:39995815
Innate and SARS-CoV-2 specific adaptive immune response kinetic in neutralizing monoclonal antibody successfully treated COVID-19 patients., PMID:39832460
An evaluation of vilobelimab (anti-C5a) as a cost-effective option to treat severely ill mechanically ventilated patients with COVID-19., PMID:39475087
Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: A Multicentric Retrospective Study., PMID:39459877
Characterisation of the antibody-mediated selective pressure driving intra-host evolution of SARS-CoV-2 in prolonged infection., PMID:39405332
Adverse events associated with SARS-CoV-2 neutralizing monoclonal antibodies using the FDA adverse event reporting system database., PMID:39345748
Identification of antibody-resistant SARS-CoV-2 mutants via N4-Hydroxycytidine mutagenesis., PMID:39293594
Impact analysis of SARS-CoV-2 vaccination in patients treated with monoclonal antibodies: A monocentric experience., PMID:39265354
Impact of SARS-CoV-2 Resistance to Antiviral Monoclonal Antibody Therapy on Neutralizing Antibody Response., PMID:39247686
Monoclonal antibodies against SARS-CoV-2 to prevent COVID-19 worsening in a large multicenter cohort., PMID:39247344
Efficacy and Safety of Low-Dose, Rapidly Infused Bamlanivimab and Etesevimab: Phase 3 BLAZE-1 Trial for Mild-to-Moderate COVID-19., PMID:39230829
Comparison Study of the Bio-Plex and Meso Scale Multiplexed SARS-CoV-2 Serology Assays Reveals Evidence of Diminished Host Antibody Responses to SARS-CoV-2 after Monoclonal Antibody Treatment., PMID:39165724
Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities., PMID:39126484
Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports., PMID:39096169
Longitudinal importance of the soluble receptor for advanced glycation end-products in nonintubated hospitalized patients with COVID-19 pneumonia., PMID:39076084
Clinical outcomes in patients with mild to moderate coronavirus disease 2019 treated with monoclonal antibody therapy versus an untreated control cohort., PMID:39066463
SARS-CoV-2 Monoclonal Antibody Treatment Followed by Vaccination Shifts Human Memory B-Cell Epitope Recognition, Suggesting Antibody Feedback., PMID:39036987
Sotrovimab in the treatment of coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis of randomized clinical trials., PMID:39031183
Safety and Efficacy of SAB-185 for Nonhospitalized Adults With COVID-19: A Randomized Clinical Trial., PMID:39028902
Real-world effectiveness of sotrovimab in preventing hospitalization and mortality in high-risk patients with COVID-19 in the United States: A cohort study from the Mayo Clinic electronic health records., PMID:39012863
Repurposing of therapeutic antibodies against dengue virus envelope protein receptor binding domain., PMID:38900285
Early trajectories of virological and immunological biomarkers and clinical outcomes in patients admitted to hospital for COVID-19: an international, prospective cohort study., PMID:38815595
Comparison of bamlanivimab with or without etesevimab and casirivimab-imdevimab in clinical outcomes in patients with COVID-19: a systematic review and meta-analysis., PMID:38721976
Viral Kinetics Model of SARS-CoV-2 Infection Informs Drug Discovery, Clinical Dose, and Regimen Selection., PMID:38676291
Efficacy and safety of bamlanivimab in patients with COVID-19: A systematic review and meta-analysis., PMID:38616851
Failure of bamlanivimab with selection of E484K mutation in an allogeneic stem cell transplant recipient with nosocomial SARS-CoV-2 infection., PMID:38353416
Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab or Sotrovimab on Persistently High Viral Load in Patients with Mild-to-Moderate COVID-19: A Randomized, Phase 2 BLAZE-4 Trial., PMID:38291279
Developing of SARS-CoV-2 fusion protein expressed in E. coli Shuffle T7 for enhanced ELISA detection sensitivity - an integrated experimental and bioinformatic approach., PMID:38234051
The Therapeutic Monoclonal Antibody Bamlanivimab Does Not Enhance SARS-CoV-2 Infection by FcR-Mediated Mechanisms., PMID:38133292
Randomized trial of the safety and efficacy of anti-SARS-CoV-2 mAb in the treatment of patients with nosocomial COVID-19 (CATCO-NOS)., PMID:38058498
SARS-CoV-2 monoclonal antibody treatment followed by vaccination shifts human memory B cell epitope recognition suggesting antibody feedback., PMID:38045374
Impaired immune responses and prolonged viral replication in lung allograft recipients infected with SARS-CoV-2 in the early phase after transplantation., PMID:37922037
The Efficacy and Safety of Monoclonal Antibody Treatments Against COVID-19: A Systematic Review and Meta-analysis of Randomized Clinical Trials., PMID:37915159
Assessment of neutralization susceptibility of Omicron subvariants XBB.1.5 and BQ.1.1 against broad-spectrum neutralizing antibodies through epitopes mapping., PMID:37828918
Modeling the emergence of viral resistance for SARS-CoV-2 during treatment with an anti-spike monoclonal antibody., PMID:37745410
Antiviral Drugs and Vaccines for Omicron Variant: A Focused Review., PMID:37719798
Evolution of a globally unique SARS-CoV-2 Spike E484T monoclonal antibody escape mutation in a persistently infected, immunocompromised individual., PMID:37692895
A Comparison of the Adverse Effects and Utility of Different Monoclonal Antibodies for SARS-CoV-2: A Retrospective Cohort Study., PMID:37680398
Long COVID After Bamlanivimab Treatment., PMID:37650236
Use of Monoclonal Antibodies in Pregnant Women Infected by COVID-19: A Case Series., PMID:37630512
Clinical Features and Risk Stratification of Multiple Myeloma Patients with COVID-19., PMID:37509261
Preliminary Evidence of Good Safety Profile and Outcomes of Early Treatment with Tixagevimab/Cilgavimab Compared to Previously Employed Monoclonal Antibodies for COVID-19 in Immunocompromised Patients., PMID:37371635
Pharmacokinetics, Efficacy, and Safety of a SARS-CoV-2 Antibody Treatment in Pediatric Participants: An Open-Label Addendum of a Placebo-Controlled, Randomized Phase 2/3 Trial., PMID:37329415
Neutralizing anti-spike monoclonal antibodies for COVID-19 in vulnerable populations: lessons learned and future directions., PMID:37318043
Outcomes of Bebtelovimab Therapy in Patients With Solid Organ Transplantation With Mild and Moderate COVID-19., PMID:37313067