Inebilizumab: First Approval, PMID: 32729016
Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial, PMID: 31495497
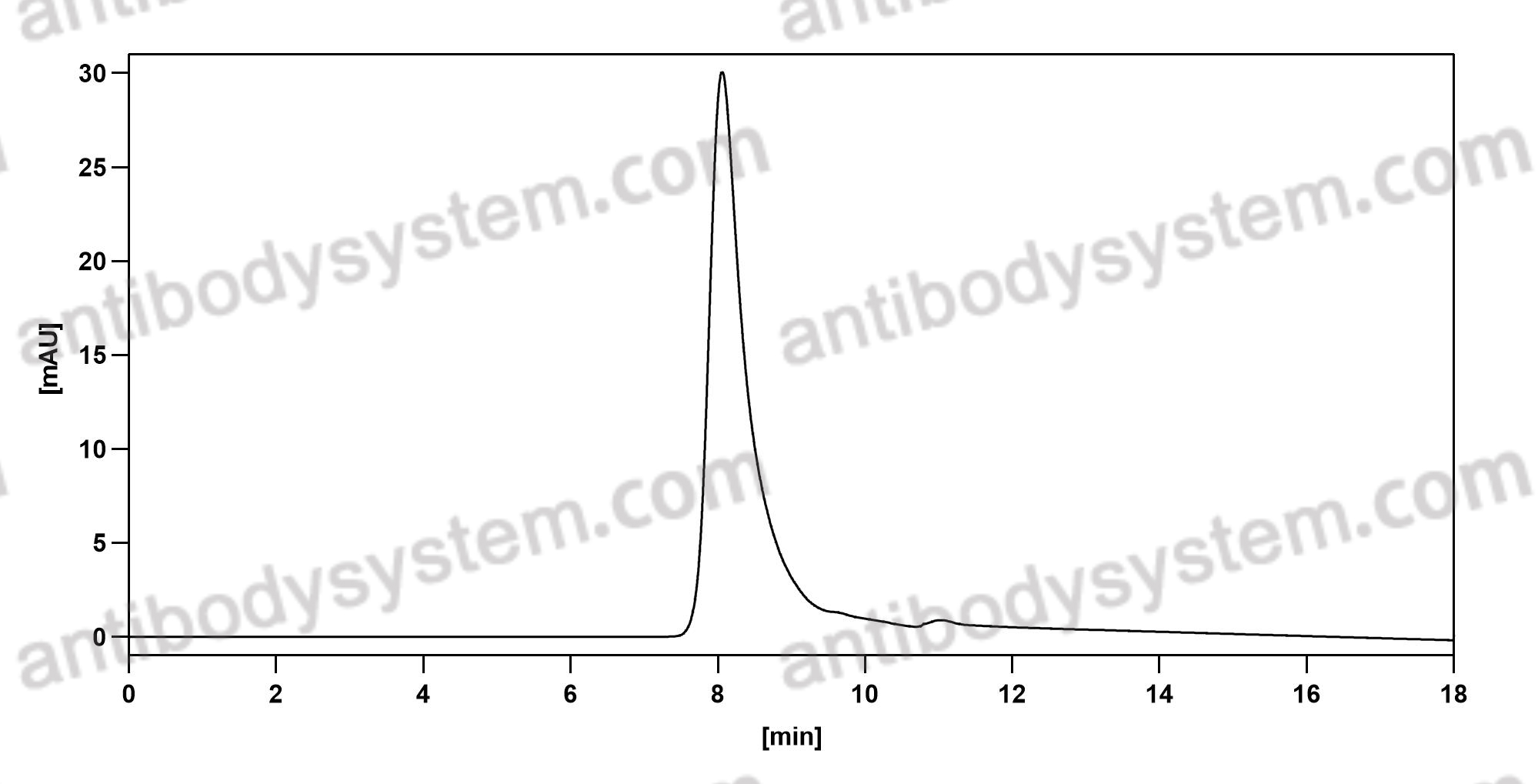
Inebilizumab-cdon, PMID: 32936251
Antibodies to watch in 2020, PMID: 31847708
Advances in the Treatment of Neuromyelitis Optica Spectrum Disorder, PMID: 33223088
Inebilizumab, a B Cell-Depleting Anti-CD19 Antibody for the Treatment of Autoimmune Neurological Diseases: Insights from Preclinical Studies, PMID: 27886126
New therapies for neuromyelitis optica spectrum disorder, PMID: 33186537
Inebilizumab in AQP4-Ab-positive neuromyelitis optica spectrum disorder, PMID: 34061127
Targeting B cells to modify MS, NMOSD, and MOGAD: Part 2, PMID: 33411674
Recent progress in maintenance treatment of neuromyelitis optica spectrum disorder, PMID: 33011853
Targeting B Cells to Modify MS, NMOSD, and MOGAD: Part 1, PMID: 33406479
Review of approved NMO therapies based on mechanism of action, efficacy and long-term effects, PMID: 33059216
Update on neuromyelitis optica spectrum disorder, PMID: 33009077
Immunotherapy in myasthenia gravis in the era of biologics, PMID: 30573759
Emerging drugs for the treatment of neuromyelitis optica, PMID: 32731771
ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52), PMID: 29447988
Clinical, Radiologic, and Prognostic Features of Myelitis Associated With Myelin Oligodendrocyte Glycoprotein Autoantibody, PMID: 30575890
A multicenter phase I study of inebilizumab, a humanized anti-CD19 monoclonal antibody, in Japanese patients with relapsed or refractory B-cell lymphoma and multiple myeloma, PMID: 30915717
Novel insights into pathophysiology and therapeutic possibilities reveal further differences between AQP4-IgG- and MOG-IgG-associated diseases, PMID: 32304439
Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: Results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study, PMID: 29143550
Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS, PMID: 33372052
Inebilizumab-cdon: USFDA approval for the treatment of NMOSD (neuromyelitis optica spectrum disorder), PMID: 34011260
Baseline Plasma Cell Gene Signature Predicts Improvement in Systemic Sclerosis Skin Scores Following Treatment With Inebilizumab (MEDI-551) and Correlates With Disease Activity in Systemic Lupus Erythematosus and Chronic Obstructive Pulmonary Disease, PMID: 29956883
Disability Outcomes in the N-MOmentum Trial of Inebilizumab in Neuromyelitis Optica Spectrum Disorder, PMID: 33771837
Sensitivity analysis of the primary endpoint from the N-MOmentum study of inebilizumab in NMOSD, PMID: 33538237
Emerging Targeted Therapies for Neuromyelitis Optica Spectrum Disorders, PMID: 33301078
Current and emerging biologics for the treatment of neuromyelitis optica spectrum disorders, PMID: 32228250
B cells in multiple sclerosis therapy-A comprehensive review, PMID: 29512131
Immune reconstitution therapy in NMOSD, PMID: 33992916
Lupus and NMOSD: The Blending of Humoral Autoimmunity, PMID: 33123402
Satralizumab in the treatment of neuromyelitis optica spectrum disorder, PMID: 33167776
Efficiency of antibody therapy in demyelinating diseases, PMID: 28910968
Investigational drugs in development to prevent neuromyelitis optica relapses, PMID: 29465257
Long-term Outcomes in Patients With Myelin Oligodendrocyte Glycoprotein Immunoglobulin G-Associated Disorder, PMID: 32865549
Efficacy and Safety of Monoclonal Antibody Therapy in Neuromyelitis Optica Spectrum Disorders: Evidence from Randomized Controlled Trials, PMID: 32442886
New Therapeutic Landscape in Neuromyelitis Optica, PMID: 33814893
Coexistence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4 Antibodies in Adult and Pediatric Patients, PMID: 31657826
B Cells and Antibodies as Targets of Therapeutic Intervention in Neuromyelitis Optica Spectrum Disorders, PMID: 33419217
[Neuromyelitis Optica Spectrum Disorder], PMID: 34006678
Recent advances in the treatment of neuromyelitis optica spectrum disorders, PMID: 33741809
Application of 2015 Seronegative Neuromyelitis Optica Spectrum Disorder Diagnostic Criteria for Patients With Myelin Oligodendrocyte Glycoprotein IgG-Associated Disorders, PMID: 32777005
Seroprevalence and clinical phenotype of MOG-IgG-associated disorders in Sri Lanka, PMID: 31387865
Drug Treatment of Neuromyelitis Optica Spectrum Disorders: Out with the Old, in with the New?, PMID: 33777853
Targeting Neuromyelitis Optica Pathogenesis: Results from Randomized Controlled Trials of Biologics, PMID: 33909234
Rituximab for the Treatment of Neuromyelitis Optica Spectrum Disorder [Internet], PMID: 34165925
Clinical Utility of Antiretinal Antibody Testing, PMID: 33885761
Clinical spectrum of high-titre GAD65 antibodies, PMID: 33563803
A lifetime aging study of human CD19 transgenic mice, PMID: 28243835
Serum Glial Fibrillary Acidic Protein: A Neuromyelitis Optica Spectrum Disorder Biomarker, PMID: 33724534
The frequency of longitudinally extensive transverse myelitis in MS: A population-based study, PMID: 31707235
Emerging Role of Targeted Monoclonal Antibodies in Neuromyelitis Optica Spectrum Disorders., PMID:40506618
An updated, comprehensive meta-analysis of the treatment of anti-NMDAR encephalitis: Analysis, equipoise, and the urgent need for evidence over anecdote., PMID:40472799
Relapses during treatment with monoclonal antibodies targeting B-cells in NMOSD., PMID:40383834
Clinical Features and Factors Associated With Outcome in Late Adult-Onset Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease., PMID:40324120
Utility of spinal cord axial-T2 sequence in a population-based cohort of multiple sclerosis., PMID:40301133
Neuromyelitis optica spectrum disorder in Latin America: a global data share initiative., PMID:40267692
A Phase 3 Trial of Inebilizumab in Generalized Myasthenia Gravis., PMID:40202593
Satralizumab after inebilizumab treatment in a patient with recurrent neuromyelitis optica spectrum disorder: A case report., PMID:40193676
Management of IgG4-related cholangitis: diagnosis, therapy, and long-term surveillance., PMID:40191403
The Evolution of Anti-CD20 Treatment for Multiple Sclerosis: Optimization of Antibody Characteristics and Function., PMID:40180777
IgG4-related disease: lessons from the first 20 years., PMID:40071397
Evaluating Rituximab Failure Rates in Neuromyelitis Optica Spectrum Disorder: A Nationwide Real-World Study From South Korea., PMID:40065454
Can Inebilizumab Change the Management of IgG4-Related Disease?, PMID:40057125
Neurite orientation dispersion and density imaging in myelin oligodendrocyte glycoprotein antibody-associated disease and neuromyelitis optica spectrum disorders., PMID:39955814
Inebilizumab treatment in a patient with co-occurring AQP4-IgG positive neuromyelitis optica spectrum disorder and myasthenia gravis: a case report and literature review., PMID:39872531
Autoimmune encephalitis: recovery, residual symptoms and predictors of long-term sequelae., PMID:39832911
Upper cervical spinal cord atrophy in MS: Sex, menopause, and neurodegeneration., PMID:39825669
MOG antibody-associated disease epidemiology in Olmsted County, USA, and Martinique., PMID:39812824
Hyperreflective retinal foci are associated with retinal degeneration after optic neuritis in neuromyelitis optica spectrum disorders and multiple sclerosis., PMID:39790055
Inebilizumab shows promise for IgG4-RD., PMID:39663465
Cerebellar Involvement in Attacks of Aquaporin-4-IgG Positive Neuromyelitis Optica Spectrum Disorder., PMID:39661938
First-in-Human Study of BAT4406F, an ADCC-Enhanced Fully Humanized Anti-CD20 Monoclonal Antibody in Patients With Neuromyelitis Optica Spectrum Disorders., PMID:39592888
Linking Downbeat Nystagmus and Aquaporin-4-Positive Neuromyelitis Optica: A Closer Look at Their Hidden Relationship., PMID:39564022
Neuromyelitis optica spectrum disorder with ultra-longitudinally extensive transverse myelitis: A case report and literature review., PMID:39559196
Inebilizumab for Treatment of IgG4-Related Disease., PMID:39541094
Recurrent late-onset neutropenia following treatment with different B cell-depleting strategies in multiple sclerosis., PMID:39515320
Usage status of biologics for the chronic treatment of optic neuritis in neuromyelitis optica spectrum disorders in Japan., PMID:39470886
Teprotumumab for the Treatment of Thyroid Eye Disease: Why Should We Keep Our Eyes "Wide Open"?-A Clinical and Pharmacovigilance Point of View., PMID:39452535
CD19: a promising target for systemic sclerosis., PMID:39421745
[Efficacy and Safety of Inebilizumab, an Anti-CD19 Monoclonal Antibody, for the Treatment of Neuromyelitis Optica Spectrum Disorder: Based on the N-MOmentum Trial]., PMID:39370840
B-cell Depletion Therapy in Pediatric Neuroinflammatory Disease., PMID:39259430
Rapid differentiation of MOGAD and MS after a single optic neuritis., PMID:39249105
Diagnostic Value of Inter-Eye Difference Metrics on OCT for Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis., PMID:39231384
B cell and aquaporin-4 antibody relationships with neuromyelitis optica spectrum disorder activity., PMID:39222408
A rare adverse effect in inebilizumab therapy for neuromyelitis optica spectrum disorder: a case report., PMID:39072007
Protocol of a prospective multicenter study on comorbidity impact on multiple sclerosis and antibody-mediated diseases of the central nervous system (COMMIT)., PMID:39021565
Seizure-Related Falls and Near Falls in LGI1-IgG Autoimmune Encephalitis., PMID:38938695
Frequency of Asymptomatic Optic Nerve Enhancement in 203 Patients With MOG Antibody-Associated Disease., PMID:38924706
Clinical features and outcomes in primary nervous system histiocytic neoplasms., PMID:38902249
Longitudinal change of serum NfL as disease activity biomarker candidate in MOGAD: A descriptive cohort study., PMID:38901371
A case report of AQP4-IgG-seropositive refractory neuromyelitis optica spectrum disorder patient with Sjögren's syndrome and pancytopenia treated with inebilizumab., PMID:38899058
Comparison of inebilizumab or rituximab in addition to glucocorticoid therapy for neuromyelitis optica spectrum disorders., PMID:38895668
Sex as a predictor of clinical phenotype and determinant of immune response in IgG4-related disease: a retrospective study of patients fulfilling the American College of Rheumatology-European League Against Rheumatism classification criteria., PMID:38824935
B cell-targeting chimeric antigen receptor T cells as an emerging therapy in neuroimmunological diseases., PMID:38760099
Safety and efficacy of inebilizumab for the treatment of neuromyelitis optica spectrum disorder: end-of-study results from the open-label period of the N-MOmentum trial., PMID:38760098
Network Meta-analysis of Ravulizumab and Alternative Interventions for the Treatment of Neuromyelitis Optica Spectrum Disorder., PMID:38722571
Radiologic Lag and Brain MRI Lesion Dynamics During Attacks in MOG Antibody-Associated Disease., PMID:38710000
Case report: Transition from anti-CD20 therapy to inebilizumab for 14 cases of neuromyelitis optica spectrum disorder., PMID:38689876