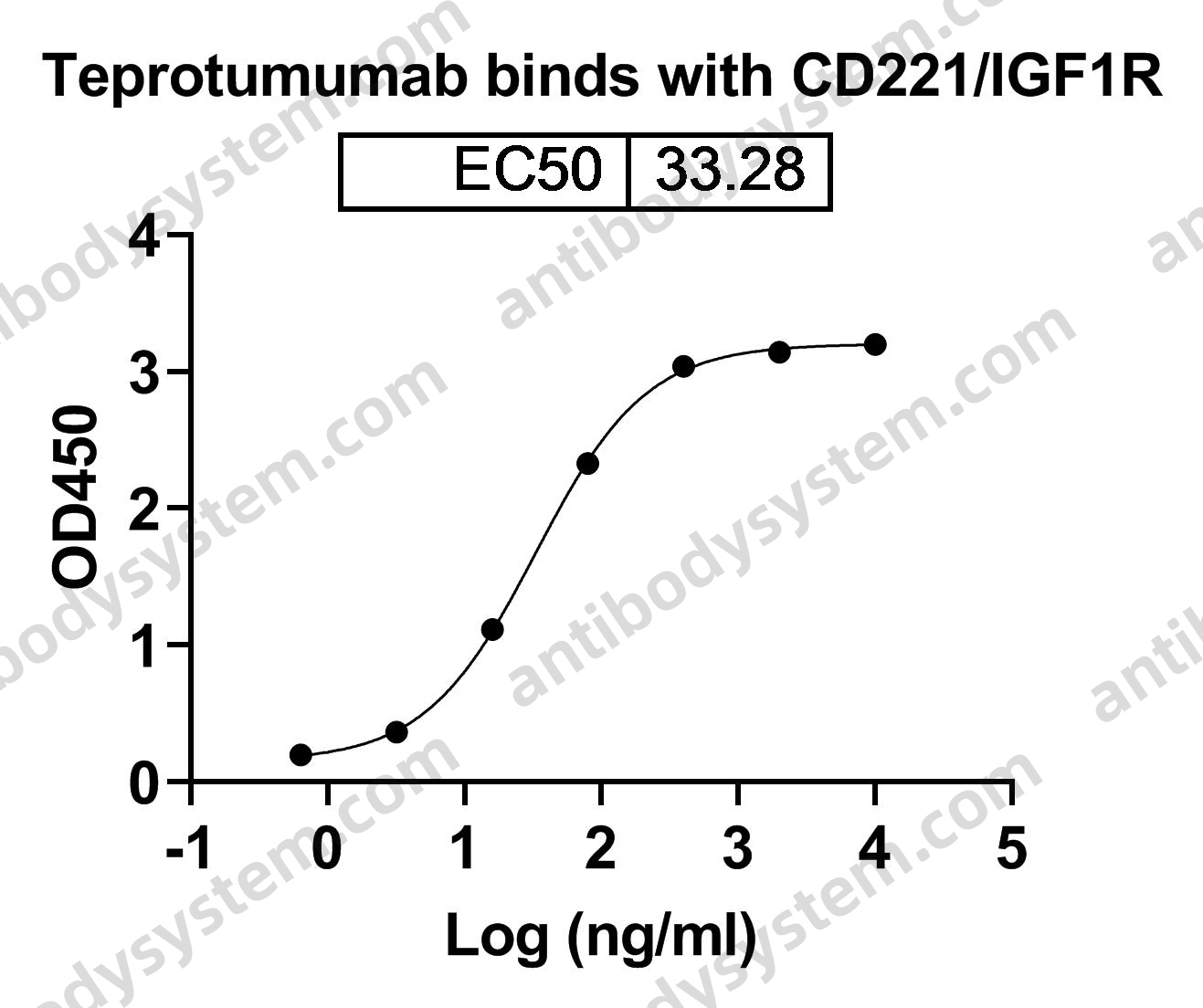
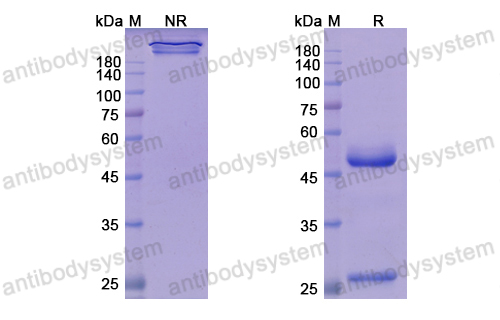
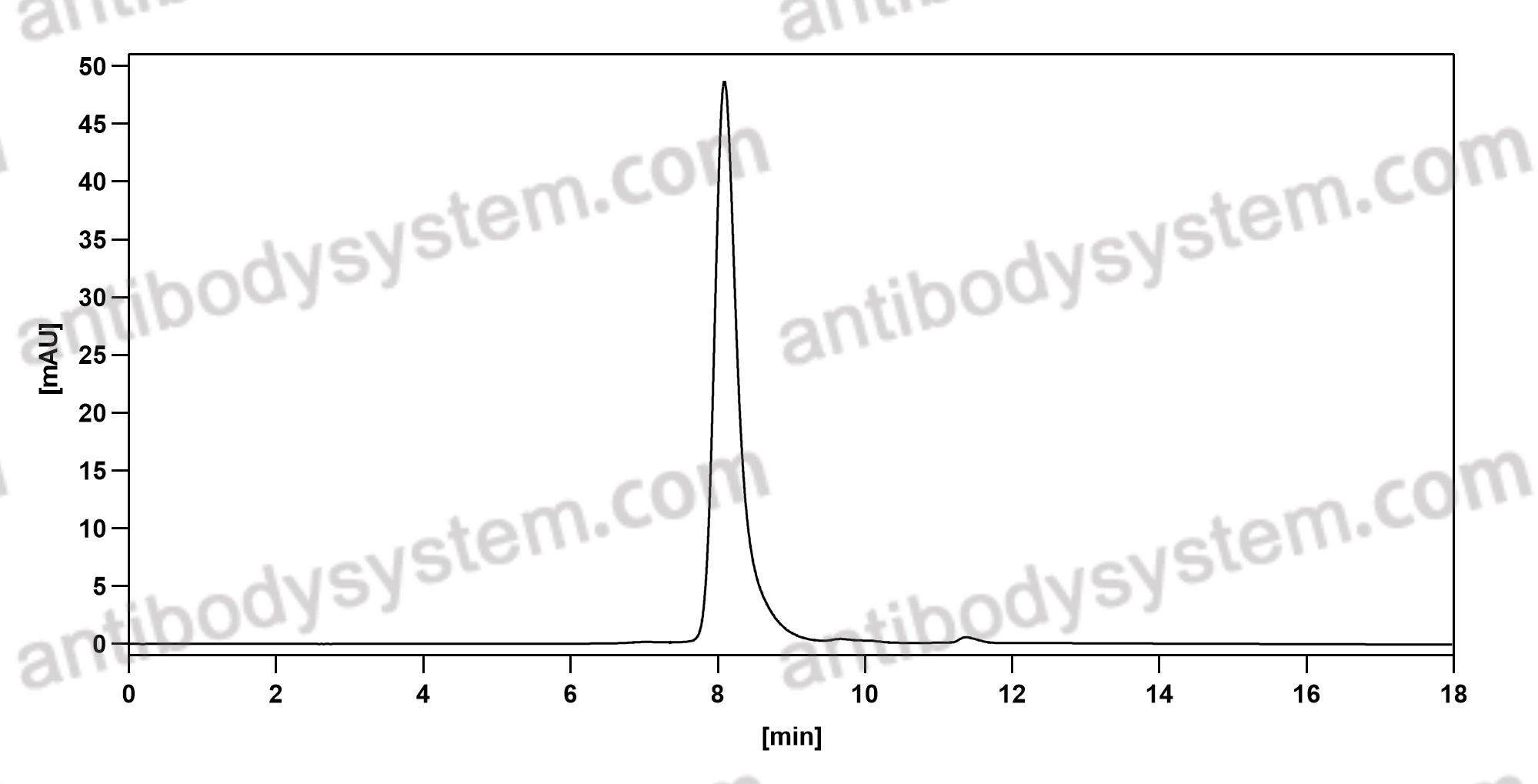
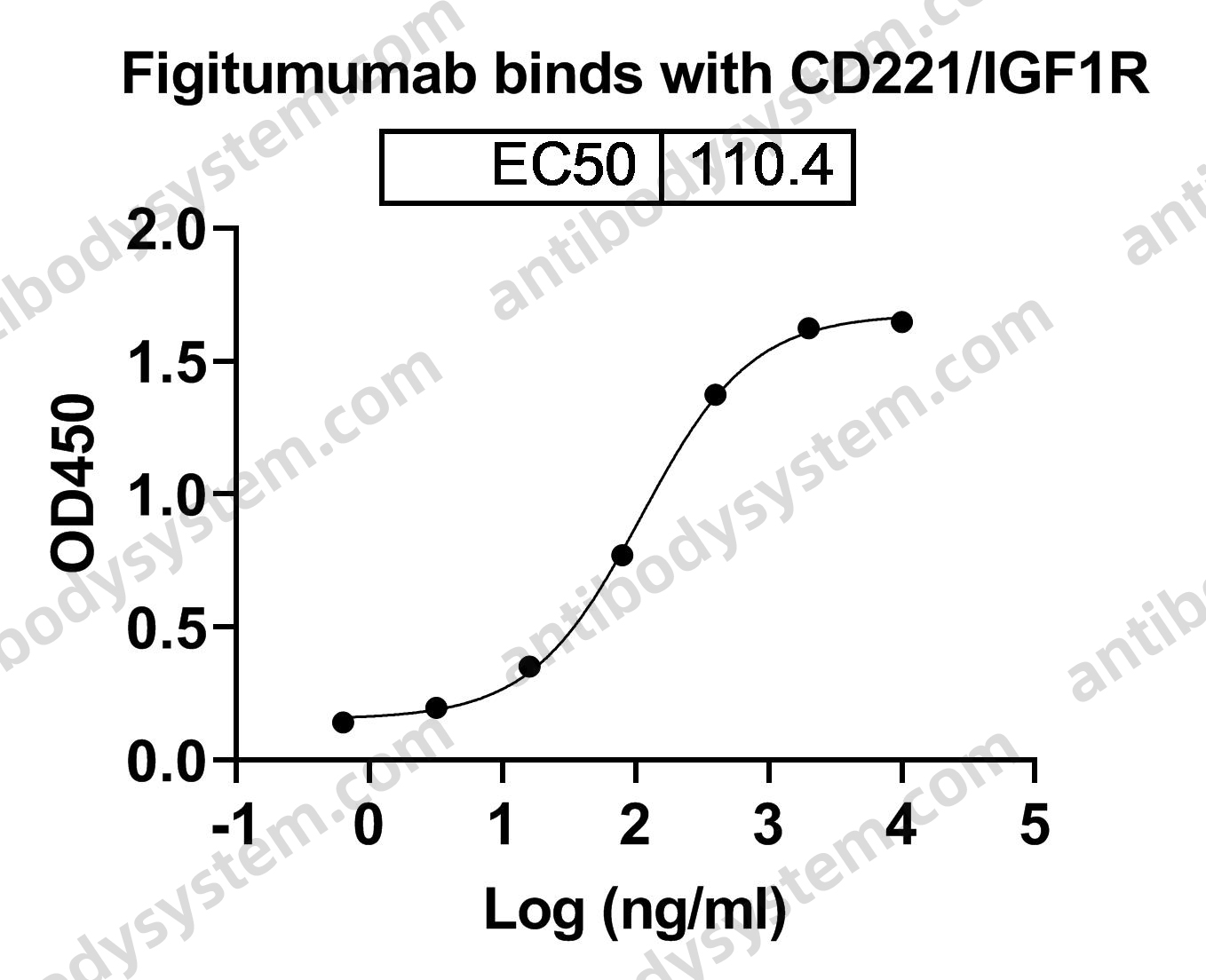
Teprotumumab: First Approval, PMID: 32157641
Teprotumumab for the Treatment of Active Thyroid Eye Disease, PMID: 31971679
Teprotumumab for Thyroid-Associated Ophthalmopathy, PMID: 28467880
Teprotumumab: a novel therapeutic monoclonal antibody for thyroid-associated ophthalmopathy, PMID: 32429706
Teprotumumab as a Novel Therapy for Thyroid-Associated Ophthalmopathy, PMID: 33391187
Teprotumumab in Thyroid-Associated Ophthalmopathy: Rationale for Therapeutic Insulin-Like Growth Factor-I Receptor Inhibition, PMID: 32040069
Teprotumumab for the treatment of thyroid eye disease, PMID: 32707005
Teprotumumab for Thyroid Eye Disease, PMID: 32205652
Teprotumumab for Active Thyroid Eye Disease, PMID: 32402171
Teprotumumab for Active Thyroid Eye Disease, PMID: 32402170
Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression, PMID: 33221815
Teprotumumab reduces extraocular muscle and orbital fat volume in thyroid eye disease, PMID: 33172865
[Teprotumumab for thyroid-associated ophthalmopathy : OPTIC], PMID: 33025123
Antibodies to watch in 2020, PMID: 31847708
Teprotumumab for Active Thyroid Eye Disease. Reply, PMID: 32402172
Teprotumumab: a disease modifying treatment for graves' orbitopathy, PMID: 32636936
Thyroid-associated ophthalmopathy: Emergence of teprotumumab as a promising medical therapy, PMID: 32088116
Treatment of Graves' ophthalmopathy, PMID: 33069387
Teprotumumab in Clinical Practice: Recommendations and Considerations From the OPTIC Trial Investigators, PMID: 33417417
Teprotumumab, PMID: 34292697
Teprotumumab (Tepezza): from the discovery and development of medicines to USFDA approval for active thyroid eye disease (TED) treatment, PMID: 33481154
Teprotumumab, PMID: 33886177
Early experience with teprotumumab for chronic thyroid eye disease, PMID: 32462101
[Teprotumumab - selective IGF-1-receptor blockade improves significantly endocrine opthalmopathy in Basedow's disease], PMID: 32392608
Teprotumumab for Dysthyroid Optic Neuropathy: Early Response to Therapy, PMID: 32976335
Current and Emerging Treatment Strategies for Graves' Orbitopathy, PMID: 30659423
Antibodies to watch in 2019, PMID: 30516432
Medical treatment in thyroid eye disease in 2020, PMID: 32447327
Ototoxicity and Teprotumumab, PMID: 34448414
New insights into the pathogenesis and nonsurgical management of Graves orbitopathy, PMID: 31889140
Teprotumumab, an insulin-like growth factor-1 receptor antagonist antibody, in the treatment of active thyroid eye disease: a focus on proptosis, PMID: 30575804
A New Era in the Treatment of Thyroid Eye Disease, PMID: 31377284
Current and Future Treatments for Graves' Disease and Graves' Ophthalmopathy, PMID: 30286486
Teprotumumab for Optic Neuropathy in Thyroid Eye Disease, PMID: 33270090
Teprotumumab for thyroid eye disease: early response is not required for benefit, PMID: 34183792
Resolution of pretibial myxedema with teprotumumab in a patient with Graves disease, PMID: 33294564
Teprotumumab: Interpreting the Clinical Trials in the Context of Thyroid Eye Disease Pathogenesis and Current Therapies, PMID: 33930408
Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy, PMID: 30215690
Teprotumumab Treatment for Thyroid-Associated Ophthalmopathy, PMID: 33511083
Graves' disease: Clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy, PMID: 32059832
Advances in treatment of active, moderate-to-severe Graves' ophthalmopathy, PMID: 27346786
[Update Graves' disease 2019], PMID: 30703831
Current Understanding of the Progression and Management of Thyroid Associated Orbitopathy: A Systematic Review, PMID: 31823232
Teprotumumab and Hearing Loss: Case Series and Proposal for Audiologic Monitoring, PMID: 34085994
Graves’ Disease: Complications, PMID: 25905406
Teprotumumab for chronic thyroid eye disease, PMID: 34060414
Immunotherapies for thyroid eye disease, PMID: 31356255
Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes, PMID: 24878056
Novel therapies for thyroid autoimmune diseases: An update, PMID: 31813786
Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials, PMID: 33865501
TSHR-targeting nucleic acid aptamer treats Graves' ophthalmopathy via novel allosteric inhibition. [DHC29903]
Case Report: Development of severe inflammatory orbitopathy after immune checkpoint inhibitor initiation., PMID:40529668
Teprotumumab in the management of thyroid eye disease mechanistic insights and adverse reactions: a comprehensive review., PMID:40496563
Persistent Palmar Rash Associated with Teprotumumab Treatment., PMID:40488249
Regional Differences in the Management of Thyroid Eye Disease: Results from an International Clinical Practice Survey of Endocrinologists., PMID:40432588
Comparative efficacy and safety of rituximab, tocilizumab, and teprotumumab in Graves' orbitopathy: a systematic review and meta-analysis., PMID:40404973
Long-term Cardiovascular, Renal, and Safety Outcomes of Teprotumumab versus Systemic Glucocorticoids in Thyroid Eye Disease: A Target Trial Emulation., PMID:40398692
Thyroid eye disease in the biologic era: a 40-year paradigm shift in nonsurgical therapeutic strategies., PMID:40397653
Teprotumumab Use in Thyroid Eye Disease: Clinical Outcomes in the United Arab Emirates- a First Regional Case Series., PMID:40396469
Time to improvement following teprotumumab treatment of thyroid eye disease: real world experience., PMID:40366381
LSD1 induces H3 K9 demethylation to promote adipogenesis in thyroid-associated ophthalmopathy., PMID:40340927
Thyroid eye disease (Graves' orbitopathy): clinical presentation, epidemiology, pathogenesis, and management., PMID:40324443
Thyroid-related orbitopathy: clinical overview, novel medical treatments and the role of orbital surgery., PMID:40317422
Statins and Thyroid Eye Disease: A Propensity Score-Matched Retrospective Cohort Analysis., PMID:40309510
Efficacy and safety of Tocilizumab for thyroid eye disease: a systemic review and Meta-analysis., PMID:40304985
Teprotumumab Improves Quality of Life in Thyroid Eye Disease: Meta-analysis and Matching-adjusted Indirect Comparison., PMID:40303547
Orbital Decompression in the Biologic Era: Is There Still a Need for Surgery?, PMID:40299895
The role of IL-6 in thyroid eye disease: an update on emerging treatments., PMID:40297767
Drug-induced hearing loss: a real-world pharmacovigilance study using the FDA adverse event reporting system database., PMID:40188564
Targeted immunotherapies for Graves' thyroidal & orbital diseases., PMID:40145088
Multiple pseudoprogressions during ongoing immunotherapy-based treatment of advanced gastric neuroendocrine carcinoma: A case report and review of literature., PMID:40092963
Spectral-domain optical coherence tomography imaging findings in patients receiving teprotumumab for thyroid eye disease., PMID:40083365
Nationwide orbital decompression volume, surgical approach, and subspecialty distribution patterns within the center for medicare and medicaid services population in the era of teprotumumab., PMID:40079573
Efficacy and Safety of Teprotumumab in Thyroid Eye Disease: A Systematic Review and Meta-Analysis., PMID:39952471
Otologic Symptoms Following Teprotumumab Administration in Patients with Thyroid Eye Disease., PMID:39951668
Thyroid dermopathy and thyroid eye disease associated with hypofunctional autoimmune thyroid disease with high TSI/anti-TSHR antibodies improved with teprotumumab., PMID:39950657
Controversies Surrounding IGF-I Receptor Involvement in Thyroid-Associated Ophthalmopathy., PMID:39909461
A randomised, double-masked, placebo-controlled trial evaluating the efficacy and safety of teprotumumab for active thyroid eye disease in Japanese patients., PMID:39896230
Novel perspectives on the pharmacological treatment of thyroid-associated ophthalmopathy., PMID:39872310
Re: Ugradar et al.: The rate of re-treatment in patients treated with teprotumumab: a multicenter study of 119 patients with 1 year of follow-up (Ophthalmology. 2025;132:92-97)., PMID:39818625
Observational Characterization of the Retreatment Course of Patients With Thyroid Eye Disease., PMID:39780310
Reply Re: "Teprotumumab for the Treatment of Recalcitrant Thyroid Eye Disease"., PMID:39752221
Re: "Teprotumumab for the Treatment of Recalcitrant Thyroid Eye Disease"., PMID:39752220
Linsitinib inhibits proliferation and induces apoptosis of both IGF-1R and TSH-R expressing cells., PMID:39723207
Clinical and Radiologic Predictors of Response to Teprotumumab: A 3D Volumetric Analysis of 35 Patients., PMID:39714282
Efficacy, Safety, and Recurrence in Older Thyroid Eye Disease Patients Undergoing Teprotumumab Treatment., PMID:39704303
Epidemiology and Management of Moderate to Severe Thyroid Eye Disease in the United States: Analysis of a Healthcare Claims Database., PMID:39690929
Comprehensive Comparisons of Different Treatments for Active Graves Orbitopathy: A Systematic Review and Bayesian Model-Based Network Meta-Analysis., PMID:39680569
Risk of audiologic side effects with teprotumumab treatment for thyroid eye disease: propensity matched analysis., PMID:39668181
Surgical Timing for Patients With Thyroid Eye Disease Treated With Teprotumumab: A Collaborative Multicenter Study., PMID:39656059
Teprotumumab versus intravenous methylprednisolone in thyroid eye disease: A systematic review., PMID:39651498
Advances of IGF-1R inhibitors in Graves' ophthalmopathy., PMID:39578269
Teprotumumab's Impact on Proptosis in Long-duration Thyroid Eye Disease: A Systematic Review and Meta-analysis., PMID:39526058
Recent advances in neuro-ophthalmology., PMID:39462921
Teprotumumab for the Treatment of Thyroid Eye Disease: Why Should We Keep Our Eyes "Wide Open"?-A Clinical and Pharmacovigilance Point of View., PMID:39452535
[Survey of the management of moderate to severe active Graves' orbitopathy at 28 metropolitan centers of excellence in France]., PMID:39368261
The Impact of Monoclonal Antibody Usage on Hearing Outcomes: A Systematic Review., PMID:39268884
Reply Re: "Proptosis Regression After Teprotumumab Treatment for Thyroid Eye Disease"., PMID:39240200
Re: "Proptosis Regression After Teprotumumab Treatment for Thyroid Eye Disease"., PMID:39240199
Effect of Cannabis Usage on Thyroid Eye Disease., PMID:39197177
Patterns of Teprotumumab-Induced Hearing Dysfunction: A Systematic Review., PMID:39194388