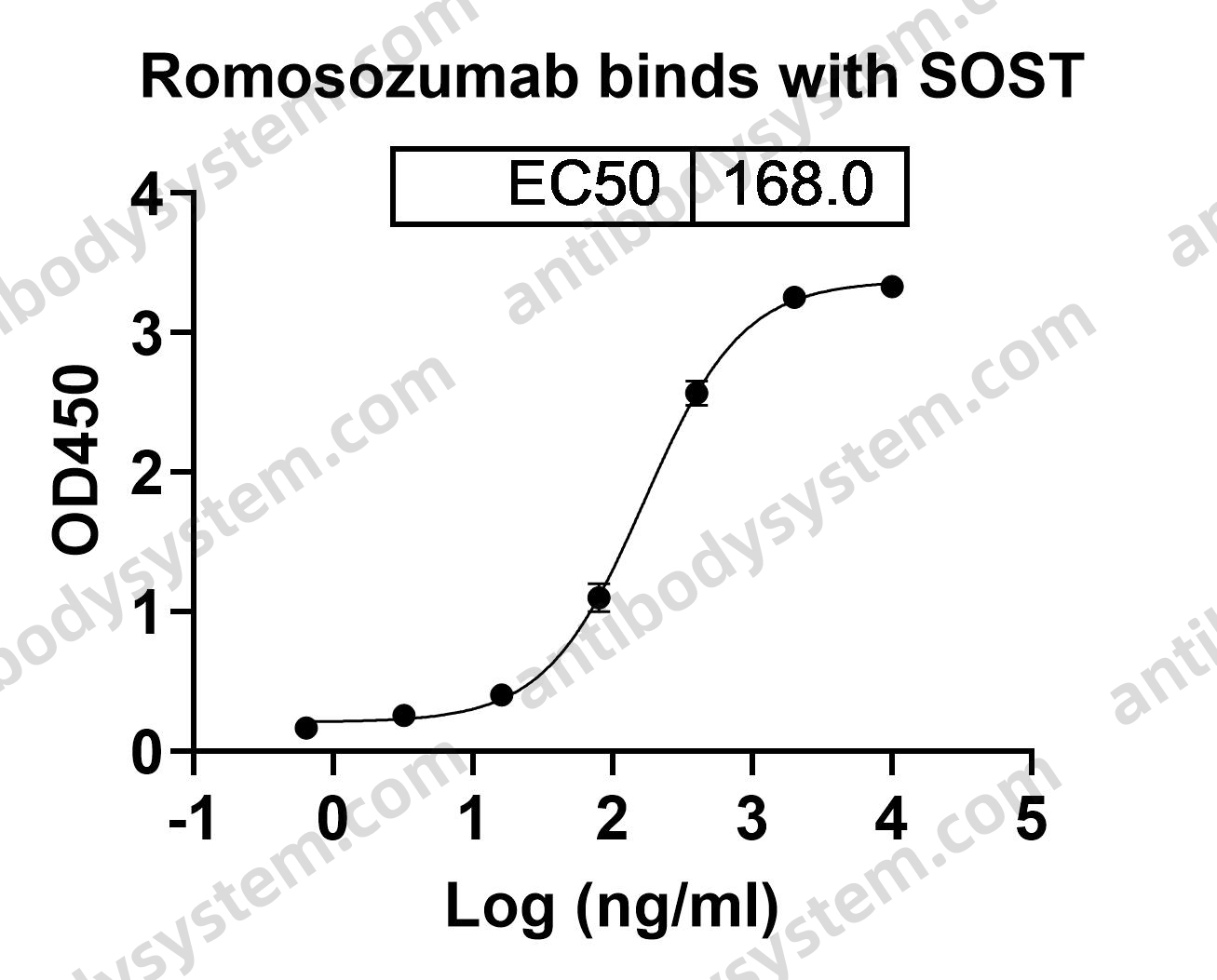
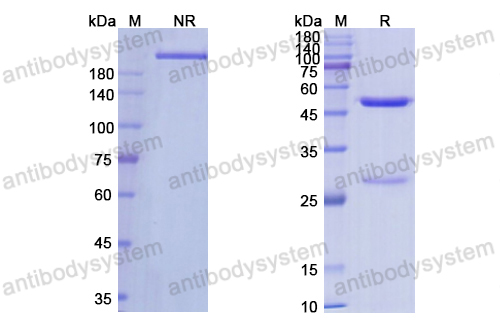
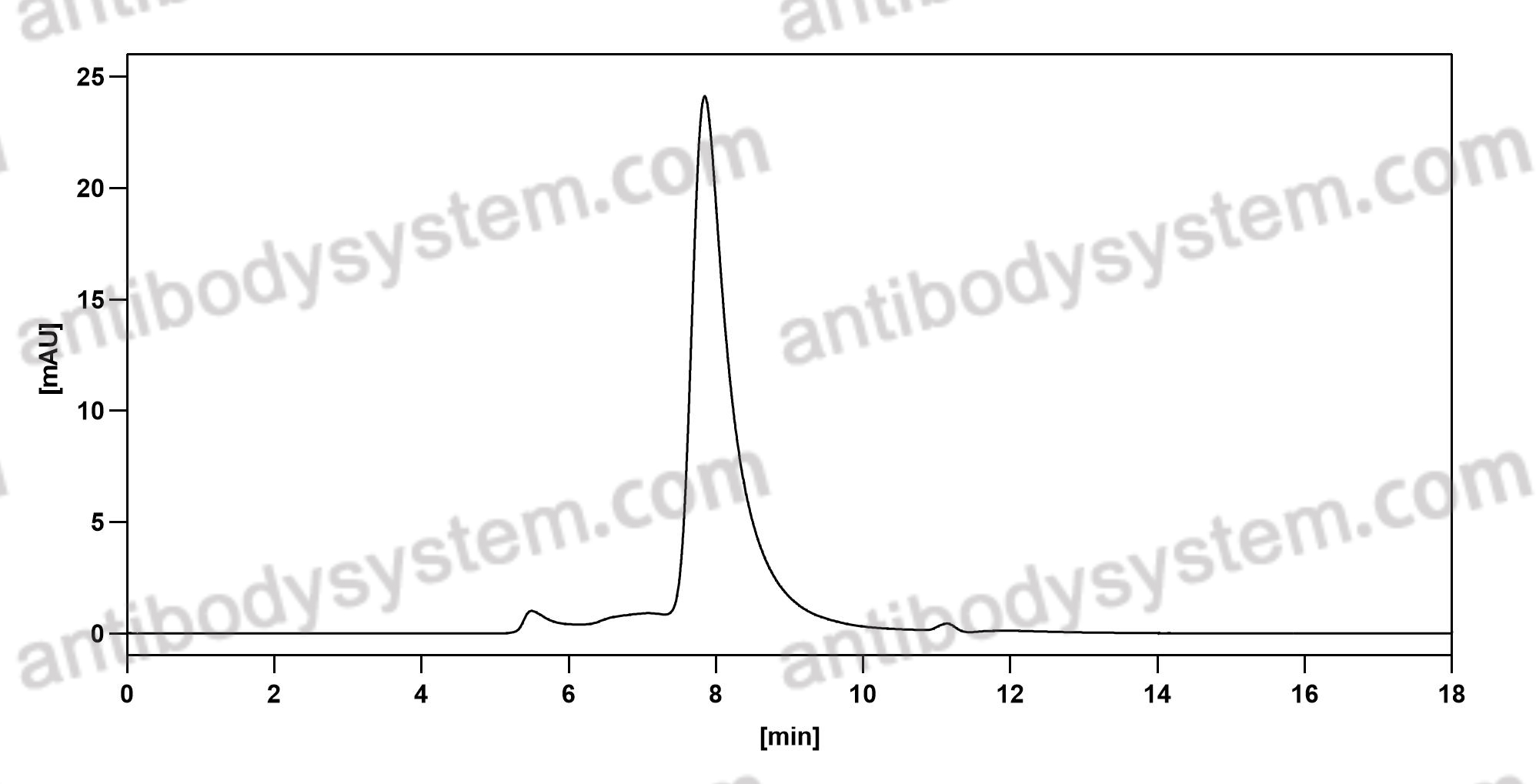
Romosozumab Treatment in Postmenopausal Women with Osteoporosis, PMID: 27641143
Romosozumab for the treatment of osteoporosis, PMID: 28064540
Romosozumab: A Review in Postmenopausal Osteoporosis, PMID: 32909197
Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis, PMID: 28892457
Romosozumab and Sequential Therapy in Postmenopausal Osteoporosis, PMID: 32600508
Romosozumab for the treatment of osteoporosis, PMID: 30775535
Romosozumab: a Review of Efficacy, Safety, and Cardiovascular Risk, PMID: 33409990
Romosozumab: A first-in-class sclerostin inhibitor for osteoporosis, PMID: 32880646
Romosozumab in postmenopausal women with low bone mineral density, PMID: 24382002
A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures, PMID: 31977817
Efficacy and safety of Romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis, PMID: 32385757
Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial, PMID: 28755782
Romosozumab: First Global Approval, PMID: 30805895
Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab, PMID: 31628490
Effects of 24 Months of Treatment With Romosozumab Followed by 12 Months of Denosumab or Placebo in Postmenopausal Women With Low Bone Mineral Density: A Randomized, Double-Blind, Phase 2, Parallel Group Study, PMID: 29694685
Romosozumab or alendronate for fracture prevention in East Asian patients: a subanalysis of the phase III, randomized ARCH study, PMID: 32047951
Cardiovascular Outcomes of Romosozumab and Protective Role of Alendronate, PMID: 31242037
Romosozumab: A Novel Injectable Sclerostin Inhibitor With Anabolic and Antiresorptive Effects for Osteoporosis, PMID: 32862655
A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis, PMID: 29931216
Bone-Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women With Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis After 2 and 12 Months of Treatment, PMID: 31233639
One Year of Romosozumab Followed by Two Years of Denosumab Maintains Fracture Risk Reductions: Results of the FRAME Extension Study, PMID: 30508316
Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: Systematic review and meta- analysis, PMID: 31678488
FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab, PMID: 29573473
Effects of Romosozumab Compared With Teriparatide on Bone Density and Mass at the Spine and Hip in Postmenopausal Women With Low Bone Mass, PMID: 27487526
Effects of prior osteoporosis treatment on early treatment response of romosozumab in patients with postmenopausal osteoporosis, PMID: 32777516
Romosozumab in the treatment of osteoporosis, PMID: 32752907
Profile of romosozumab and its potential in the management of osteoporosis, PMID: 28458516
A single dose of zoledronate preserves bone mineral density for up to 2 years after a second course of romosozumab, PMID: 32623487
Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: A systematic review and network meta-analysis, PMID: 31626995
Romosozumab followed by denosumab in Japanese women with high fracture risk in the FRAME trial, PMID: 33057807
Relationship between P1NP, a biochemical marker of bone turnover, and bone mineral density in patients transitioned from alendronate to romosozumab or teriparatide: a post hoc analysis of the STRUCTURE trial, PMID: 31707465
Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: A review, PMID: 31922699
Osteoporosis: now and the future, PMID: 21450337
Increased bone mineral density for 1 year of romosozumab, vs placebo, followed by 2 years of denosumab in the Japanese subgroup of the pivotal FRAME trial and extension, PMID: 31168657
Osteoporosis treatment: recent developments and ongoing challenges, PMID: 28689769
Early clinical effects, safety, and appropriate selection of bone markers in romosozumab treatment for osteoporosis patients: a 6-month study, PMID: 32979066
Osteoporosis in Older Adults, PMID: 32773051
Antibodies to watch in 2020, PMID: 31847708
Romosozumab-aqqg, upadacitinib, and risankizumab-rzaa, PMID: 31735342
Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation, PMID: 32588816
[The sequential therapy of romosozumab followed by denosumab for osteoporosis], PMID: 30814382
Romosozumab in Skeletally Mature Adults with a Fresh Unilateral Tibial Diaphyseal Fracture: A Randomized Phase-2 Study, PMID: 32358413
Romosozumab, clinical trials, and real-world care of patients with osteoporosis, PMID: 32953774
Safety of Romosozumab in Osteoporotic Men and Postmenopausal Women: A Meta-Analysis and Systematic Review, PMID: 32195618
Unmasking romosozumab, PMID: 28213619
Romosozumab (Evenity) for postmenopausal osteoporosis, PMID: 31170119
Romosozumab for osteoporosis, PMID: 34211250
Romosozumab, PMID: 34251779
Romosozumab was not effective in preventing multiple spontaneous clinical vertebral fractures after denosumab discontinuation: A case report, PMID: 32548215
Carcinogenicity risk assessment of romosozumab: A review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study, PMID: 27569204
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard., PMID:40529844
Romosozumab increases bone mineral density in Japanese older adults with osteoporosis., PMID:40488406
Fragility of Evidence for the Efficacy of Anti-fracture Medications., PMID:40488289
Development of a novel macroscopic regulation and microscopic intervention mode nanosystem for osteoporosis treatment., PMID:40475860
Diagnosis of skeletal fragility due to Loeys-Dietz syndrome and treatment with romosozumab followed by denosumab., PMID:40469084
Novel Aptamers Targeting Sclerostin Loop3 Improve Skeletal and Muscle Properties Without Adverse Cardiovascular Effects in Orchiectomized Mice., PMID:40464222
Association of anti-citrullinated protein antibodies with changes in bone density in patients with rheumatoid arthritis treated with romosozumab for one year: a cohort study., PMID:40459239
Treatment of Osteoporosis in Patients with Chronic Kidney Disease., PMID:40457078
Methodological concerns, implausibly early fracture reduction, and potential outcome misclassification: Comment on "Comparative effectiveness of romosozumab versus teriparatide for fracture prevention: A new-user, active comparator design"., PMID:40456504
Characteristics and treatment options of 272,152 geriatric patients with very high and high fracture risk., PMID:40445411
Role of Sclerostin in Cardiovascular System., PMID:40429697
Five-Year Sales Trends of Osteoporosis Medications in Korea: A Market Analysis Based on IMS Health Sales Audit Data (2018-2023)., PMID:40428763
Comprehensive Evaluation of Treatment Patterns in Postmenopausal Patients with Osteoporosis without Fractures: Insights from Tertiary Care Institutions and Nationwide OMOP-CDM Data., PMID:40426310
Examining Romosozumab Adherence and Side Effects in Osteoporotic Patients After Surgical Fracture Fixation: A Comparative, Descriptive, and Hypothesis-Generating Study with Non-Fractured Controls., PMID:40422580
Expression of Concern: Comparative cardiovascular safety of romosozumab versus bisphosphonates in Japanese patients with osteoporosis: a new-user, active comparator design with instrumental variable analyses., PMID:40413791
Comparative effectiveness of romosozumab versus teriparatide for fracture prevention: A new-user, active comparator design., PMID:40381877
Agents to treat osteoporosis in chronic kidney disease., PMID:40377654
Impact of Frailty and Other Factors as Estimated by HU to Predict Response to Anabolic Bone Medications., PMID:40364278
Real-World Evaluation of 12-Month Romosozumab Treatment in Korean Women with Severe Osteoporosis: Potential Synergy with Hormone Therapy., PMID:40363988
Cost-effectiveness of opportunistic osteoporosis screening using chest radiographs with deep learning in Germany., PMID:40355760
Romosozumab for managing severe osteoporosis in patients undergoing kidney transplantation: a retrospective case series., PMID:40353207
Pregnancy and lactation-related osteoporosis associating multiple vertebral fragility fractures treated with romosozumab: a case report., PMID:40330931
Assessing the Efficacy of Romosozumab in Postmenopausal Osteoporosis: An Updated Systematic Review and Meta-analysis., PMID:40323656
National guidelines for diagnosis and treatment of osteoporosis in Slovakia., PMID:40319419
Male Osteoporosis: A Comprehensive Review of Treatment Approaches in Modern Pharmacotherapy and Traditional Chinese Medicine Interventions., PMID:40304326
Managing delayed union of fragility fractures of the pelvis successfully using romosozumab: A case report., PMID:40291407
Anti-osteoporosis medication in patients with posterior spine fusion: a systematic review and meta-analysis., PMID:40280495
Use of raloxifene as a sequential therapy after romosozumab : an observational study., PMID:40268433
[Sequential drug treatments for osteoporosis]., PMID:40241552
[Clinical efficacy of osteoporosis treatments]., PMID:40241551
Bone disease and osteoporosis associated with Pompe disease., PMID:40224915
A Commentary on "Comparative Analysis of Romosozumab Versus Vertebroplasty With Denosumab: Efficacy, Safety, and Secondary Bone Mineral Density Outcomes"., PMID:40211518
Comparative Analysis of Romosozumab Versus Vertebroplasty With Denosumab: Efficacy, Safety, and Secondary Bone Mineral Density Outcomes., PMID:40211517
Role of Anabolic Anti-Osteoporosis Therapy in Diabetes Subjects., PMID:40181849
An update on the pharmacotherapy of osteoporosis., PMID:40178951
Mechanical Analysis of Romosozumab's Effects on Bone Strength in a Rat Posterolateral Lumbar Fusion Model., PMID:40161136
Use of Teriparatide, Denosumab, and Romosozumab in a Postpartum Monogenic Osteoporosis With a WNT1 Pathogenic Variation., PMID:40144478
Predictive Factors for Increased Bone Density Following Romosozumab Administration Based on Pre-Administration Blood Test Results., PMID:40134082
Twenty-Year Trends in Osteoporosis Treatment and Post-Fracture Care in South Korea: A Nationwide Study., PMID:40098430
L5 osteotomy combined with adjuvant romosozumab therapy for L5 osteoporotic vertebral fracture-induced spinal deformity: illustrative case., PMID:40096736
[Osteoporosis therapy - Update 2025, Part 1: Antiresorptive and osteoanabolic therapy options]., PMID:40091709
[Osteoporosis therapy - Update 2025, Part 2: Sequential osteoporosis therapy]., PMID:40091708
[Risk of jaw osteonecrosis and atypical femoral fracture: how to inform patients with osteoporosis?]., PMID:40091707
Romosozumab and cardiovascular safety in Japan., PMID:40057912
Response to Letter to the Editor of JBMR: sequential osteoanabolic therapy for osteoporosis., PMID:40044117
Sequential and combination therapy with romosozumab., PMID:40024934
Misaligned cardiovascular safety warnings for romosozumab in Japan., PMID:39994044
Assessment of evidence for the off-label application of osteoanabolic drugs in fracture healing and spinal fusion., PMID:39964554
Basic and Clinical Scientists Working Together-Do We Make the Best of Both Worlds?, PMID:39953279
Optimisation of romosozumab plus denosumab sequential treatments against postmenopausal osteoporosis. Insights from in silico simulations., PMID:39951226