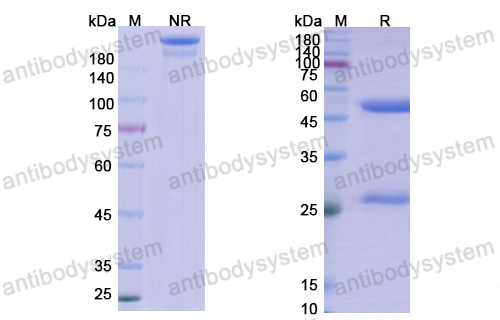
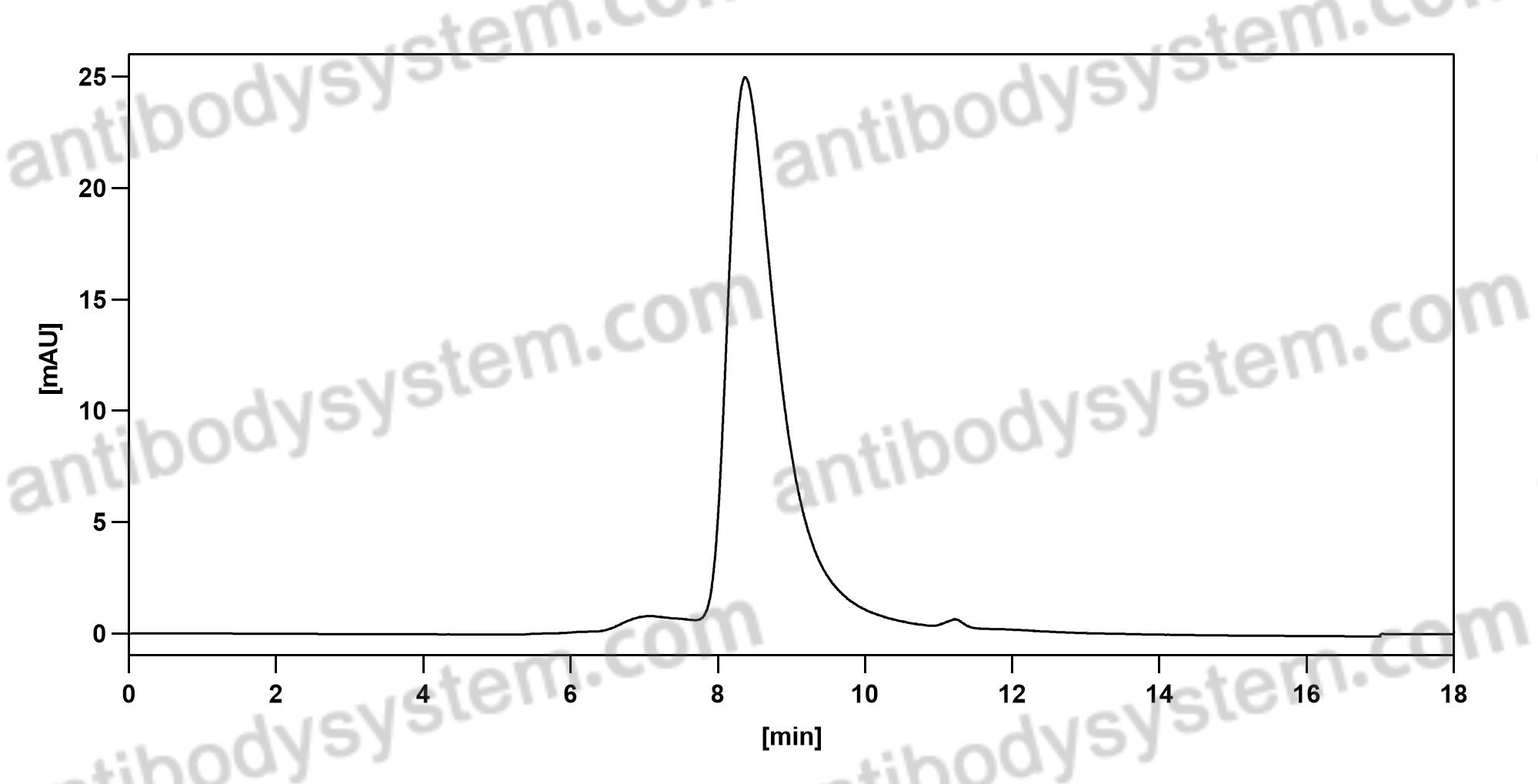
Effect of Lanadelumab Compared With Placebo on Prevention of Hereditary Angioedema Attacks: A Randomized Clinical Trial, PMID: 30480729
Lanadelumab: A Review in Hereditary Angioedema, PMID: 31560114
Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks, PMID: 32452549
Lanadelumab, PMID: 31643419
Lanadelumab: First Global Approval, PMID: 30267321
Lanadelumab to treat hereditary angioedema, PMID: 31347612
Lanadelumab for the treatment of hereditary angioedema, PMID: 31657963
Lanadelumab, PMID: 30272897
Lanadelumab for the prevention of attacks in hereditary angioedema, PMID: 31721602
Hereditary Angioedema, PMID: 32187470
Lanadelumab for the Prophylactic Treatment of Hereditary Angioedema with C1 Inhibitor Deficiency: A Review of Preclinical and Phase I Studies, PMID: 30539362
A review of kallikrein inhibitor lanadelumab in hereditary angioedema, PMID: 31234673
Lanadelumab Injection Treatment For The Prevention Of Hereditary Angioedema (HAE): Design, Development And Place In Therapy, PMID: 31695331
Pharmacoeconomic Review Report: Lanadelumab (Takhzyro): (Shire Pharma Canada ULC) [Internet], PMID: 32598112
Clinical Review Report Lanadelumab (Takhzyro): (Shire Pharma Canada ULC) [Internet], PMID: 32603039
Impact of lanadelumab on health-related quality of life in patients with hereditary angioedema in the HELP study, PMID: 33258114
Hereditary and acquired angioedema, PMID: 31690390
Pharmacokinetics, Pharmacodynamics, and Exposure-Response of Lanadelumab for Hereditary Angioedema, PMID: 32407574
Prospective Analysis in Patients With HAE Under Prophylaxis With Lanadelumab: A Real-life Experience, PMID: 33026762
Antibodies to watch in 2019, PMID: 30516432
Lanadelumab (Takhzyro) for prevention of hereditary angioedema, PMID: 33755657
CADTH Canadian Drug Expert Committee Recommendation: Lanadelumab (Takhzyro — Shire Pharma Canada ULC): Indication: For the routine prevention of attacks of hereditary angioedema (HAE) in adolescents and adults [Internet], PMID: 32574003
Does hereditary angioedema make COVID-19 worse?, PMID: 32834893
[Lanadelumab : Future plasma-independent subcutaneous prophylaxis for bradykinin-mediated angioedema?], PMID: 28560465
The Effectiveness and Value of Lanadelumab and C1 Esterase Inhibitors for Prophylaxis of Hereditary Angioedema Attacks, PMID: 30698087
Inhibiting Plasma Kallikrein for Hereditary Angioedema Prophylaxis, PMID: 28225674
Pharmacotherapy, PMID: 29777743
Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada, PMID: 34301329
An open-label study to evaluate the long-term safety and efficacy of lanadelumab for prevention of attacks in hereditary angioedema: design of the HELP study extension, PMID: 29043014
Lanadelumab Efficacy, Safety, and Injection Interval Extension in HAE: A Real-Life Study, PMID: 34023564
Efficacy of lanadelumab in acquired angioedema with C1-inhibitor deficiency, PMID: 33556593
[Monoclonal antibodies: also for dermatologists!], PMID: 31903911
Indirect Comparison of Lanadelumab and Intravenous C1-INH Using Data from the HELP and CHANGE Studies: Bayesian and Frequentist Analyses, PMID: 33646565
Transition to lanadelumab-flyo from three medications for a hereditary angioedema patient with a variant in the SYTL2 gene: A case report, PMID: 33936709
Effectiveness of lanadelumab in patients with hereditary angioedema with normal C1 inhibitor and FXII mutation, PMID: 34082125
Effectiveness of lanadelumab for preventing hereditary angioedema attacks: Subgroup analyses from the HELP study, PMID: 34166549
Pediatric hereditary angioedema: what the otolaryngologist should know, PMID: 31592791
Long-term prevention of hereditary angioedema attacks with lanadelumab: The HELP OLE Study, PMID: 34287942
Pharmaceutical Approval Update, PMID: 30559583
Biological therapy in hereditary angioedema: transformation of a rare disease, PMID: 31994957
[Monoclonal antibodies for monogenic diseases: a 2019 update], PMID: 31903913
[Hereditary angioedema due to C1-esterase inhibitor deficiency : novel approaches], PMID: 32270933
A lesson from a saboteur: High-MW kininogen impact in coronavirus-induced disease 2019, PMID: 32497257
A focus on the use of subcutaneous C1-inhibitor for treatment of hereditary angioedema, PMID: 32237900
Increased fibrinolysis-induced bradykinin formation in hereditary angioedema confirmed using stored plasma and biotechnological inhibitors, PMID: 31133046
Antibodies to watch in 2018, PMID: 29300693
Breakthroughs in hereditary angioedema management: a systematic review of approved drugs and those under research, PMID: 31645881
Antibodies to watch in 2017, PMID: 27960628
Recognition and Management of Hereditary Angioedema: Best Practices for Dermatologists, PMID: 34460082
Current medical management of hereditary angioedema: Follow-up survey of US physicians, PMID: 33122123
A national survey of four decades of hereditary angioedema prophylaxis: Efficacy and safety of old and new drugs., PMID:40513629
Sustained Effectiveness, Tolerability, and Safety of Long-Term Prophylaxis with Lanadelumab in Hereditary Angioedema: The Prospective, Phase 4, Noninterventional EMPOWER Real-World Study., PMID:40504359
Lanadelumab for prevention of attacks of non-histaminergic normal C1 inhibitor angioedema: results from the randomized, double-blind CASPIAN Study and CASPIAN open-label extension., PMID:40469312
Network Meta-Analysis of Pharmacological Therapies for Long-Term Prophylactic Treatment of Patients with Hereditary Angioedema., PMID:40434599
Hereditary angioedema plasma proteomics following specific plasma kallikrein inhibition with lanadelumab., PMID:40417315
Case Report: Identification of a novel mutation, c.1067T > A, in the SERPING1 gene in a Chinese male with type 1 hereditary angioedema., PMID:40364801
Hereditary angioedema-induced laryngeal edema: A diagnostic and therapeutic race against time., PMID:40307161
Adherence and persistence among patients with hereditary angioedema receiving long-term prophylaxis in the United States., PMID:40300843
Expert Consensus on the Diagnosis and Treatment of Hereditary Angioedema in China (2024 Edition)., PMID:40209692
[A CASE OF HEREDITARY ANGIOEDEMA WITH NORMAL C1 INHIBITOR (HEREDITARY ANGIOEDEMA TYPE III) WITH A PATHOGENIC VARIANT IN THE PLG GENE]., PMID:40204483
Comment on "Long-term prevention of hereditary angioedema attacks with lanadelumab in adolescents"., PMID:40187829
Effective long-term prophylaxis with lanadelumab in adolescents with hereditary angioedema: EMPOWER/ENABLE., PMID:40171989
Comorbidities in Canadian patients with hereditary angioedema: a quantitative survey study., PMID:40108700
Comparison of real-world healthcare resource utilization and costs among patients with hereditary angioedema on lanadelumab or berotralstat long-term prophylaxis., PMID:39976166
Analysis of prodromal symptoms and need for short-term prophylaxis in angioedema patients under long-term prophylaxis., PMID:39893484
Characteristics of Patients with Hereditary Angioedema Who Reduced Lanadelumab Treatment Administration Frequency: A Retrospective Observational Study of US Claims Data., PMID:39875773
Indirect treatment comparison of lanadelumab and a C1-esterase inhibitor in pediatric patients with hereditary angioedema., PMID:39836016
[Hereditary angioedema type 1, limitations for prophylactic therapy with Lanadelumab: Regarding a case]., PMID:39752269
Idiopathic non-mast cell angioedema: Treatment insights from global experts., PMID:39741376
Real-World Effectiveness of Lanadelumab in Hereditary Angioedema: Multicountry INTEGRATED Observational Study., PMID:39701274
Hereditary angioedema in children: Review and practical perspective for clinical management., PMID:39655944
Biologics in allergology and clinical immunology: Update on therapies for atopic diseases, urticaria, and angioedema and on safety aspects focusing on hypersensitivity reactions., PMID:39600395
Successful lanadelumab dose spacing in type I hereditary angio-oedema., PMID:39566104
The international HAE guideline under real-life conditions: From possibilities to limits in daily life - current real-world data of 8 German angioedema centers., PMID:39564138
Hereditary Angioedema Attacks in Patients Receiving Long-Term Prophylaxis: A Systematic Review., PMID:39508959
Lanadelumab in a kidney transplant patient with hereditary angioedema due to C1-inhibitor deficiency and high cardiovascular risk - a case report., PMID:39399485
A novel assay of excess plasma kallikrein-kinin system activation in hereditary angioedema., PMID:39391687
Initial Experience of Long-Term Prophylaxis with Lanadelumab for Hereditary Angioedema in China: A Clinical Observation Study on Six Patients., PMID:39362190
Angioedema due to Acquired C1-Inhibitor Deficiency Associated With Monoclonal Gammopathies of Undetermined Significance Characteristics of a French National Cohort., PMID:39357560
Real-world outcomes in patients with hereditary angioedema prescribed lanadelumab versus other prophylaxis., PMID:39293940
Long-term prevention of hereditary angioedema attacks with lanadelumab in adolescents., PMID:39128590
Hereditary angioedema prevalence and satisfaction with prophylaxis in South Australia., PMID:39006039
Androgen transition and management of hereditary angioedema long-term prophylaxis in real life: a single-center case series., PMID:38978077
An international survey assessing the effects of the duration of attack-free period on health-related quality of life for patients with hereditary angioedema., PMID:38909246
New Therapies for Type 1 and Type 2 Hereditary Angioedema., PMID:38819650
The real life experience goes on: update after 4 years on the first cohort treated with lanadelumab at our center., PMID:38799421
A quantitative systems pharmacology model of plasma kallikrein-kinin system dysregulation in hereditary angioedema., PMID:38734778
Immunogenicity of COVID-19 booster vaccination in IEI patients and their one year clinical follow-up after start of the COVID-19 vaccination program., PMID:38698851
Letter in reply: network meta-analysis for indirect comparison of lanadelumab and for the treatment of hereditary angioedema., PMID:38606574
Letter to the editor: network meta-analysis for indirect comparison of lanadelumab and berotralstat for the treatment of hereditary angioedema., PMID:38545965
Clinical Experience with Berotralstat in Patients with Hereditary Angioedema with Normal C1-Esterase Inhibitor: A Commented Case Series., PMID:38415051
Effect of lanadelumab on attack frequency and QoL in Japanese patients with hereditary angioedema: Report of five cases., PMID:38268496
A clinical evaluation of patients with known mutations (plasminogen and factor XII) with a focus on prophylactic treatment., PMID:38086754
Long-term prophylaxis for hereditary angioedema: Initial experiences with garadacimab and lanadelumab., PMID:38024849
Promising therapeutic targets for ischemic stroke identified from plasma and cerebrospinal fluid proteomes: a multicenter Mendelian randomization study., PMID:38016292
Hereditary angioedema outcomes in US patients switched from injectable long-term prophylactic medication to oral berotralstat., PMID:38006972
[Experiencia inicial de Lanadelumab en una paciente mexicana con angioedema hereditario tipo I]., PMID:37933935
Pathogenic variant in SERPING1 gene causing autosomal dominant hereditary angioedema in early childhood., PMID:37923334
Uncovering the true burden of hereditary angioedema due to C1-inhibitor deficiency: A focus on the Asia-Pacific region., PMID:37898409