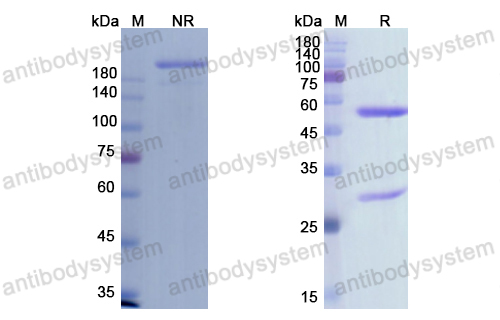
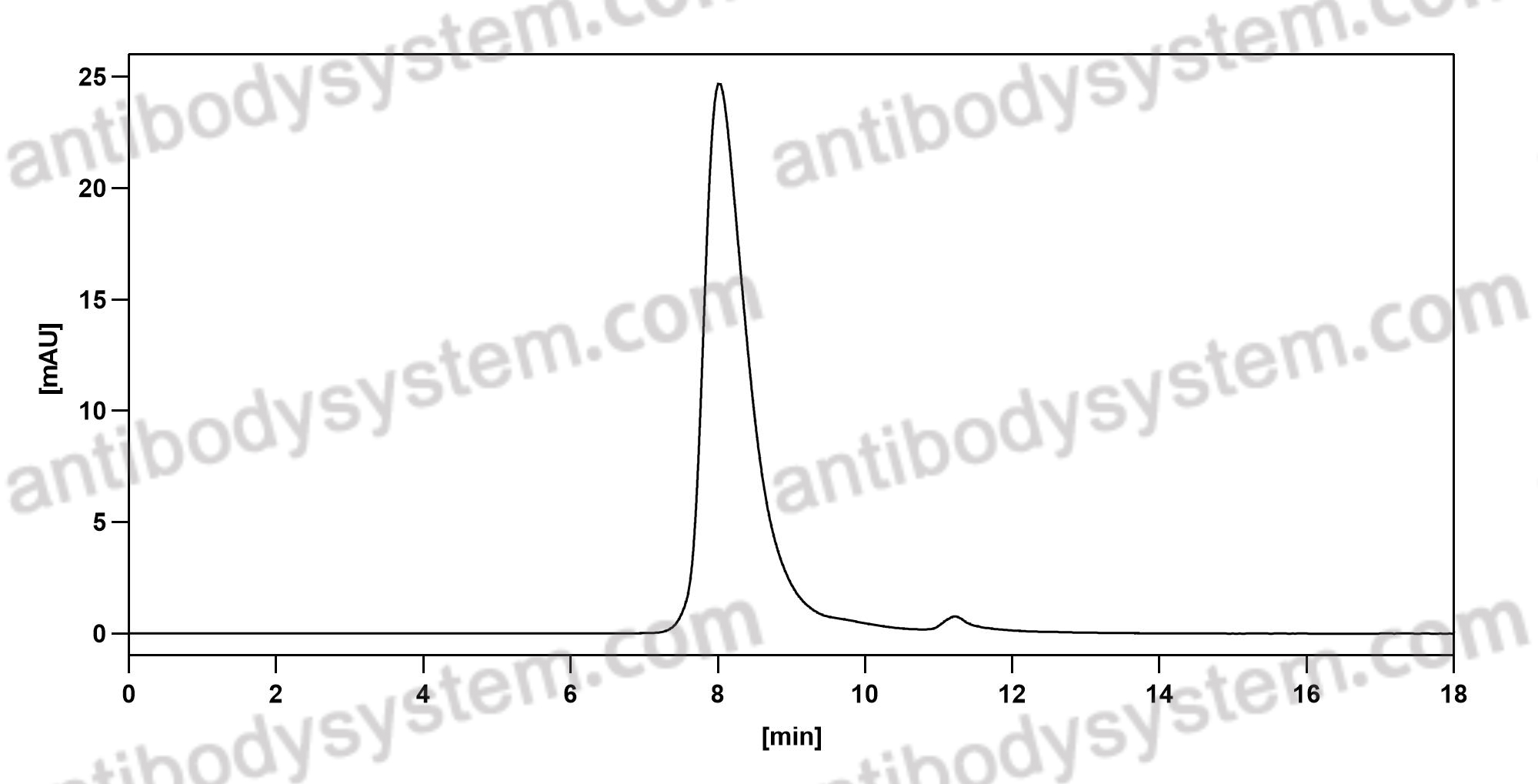
PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma, PMID: 29863979
Cemiplimab: A Review in Advanced Cutaneous Squamous Cell Carcinoma, PMID: 32306208
Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial, PMID: 31952975
Cemiplimab-rwlc as first and only treatment for advanced cutaneous squamous cell carcinoma, PMID: 31524530
Cemiplimab, PMID: 31643611
Cemiplimab, PMID: 30372008
Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing, PMID: 32554615
First-In-Human Study of Cemiplimab Alone or In Combination with Radiotherapy and/or Low-dose Cyclophosphamide in Patients with Advanced Malignancies, PMID: 31796520
Cemiplimab: First Global Approval, PMID: 30456447
PD-1 blockade in recurrent or metastatic cervical cancer: Data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer, PMID: 32917410
CASE (CemiplimAb-rwlc Survivorship and Epidemiology) study in advanced cutaneous squamous cell carcinoma, PMID: 31951149
Cemiplimab Approved for Treatment of CSCC, PMID: 30377167
Checkpoint Inhibitor-Induced Colitis, PMID: 31922959
Cemiplimab effective in cutaneous SCC, PMID: 29921845
Comparative efficacy of cemiplimab versus other systemic treatments for advanced cutaneous squamous cell carcinoma, PMID: 33052055
Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence, PMID: 32245016
Radiation recall during cemiplimab therapy for locally advanced cutaneous squamous cell carcinoma, PMID: 33068047
Cemiplimab for the treatment of advanced cutaneous squamous cell carcinoma, PMID: 31461085
Antibodies to watch in 2019, PMID: 30516432
Dose exploration results from Phase 1 study of cemiplimab, a human monoclonal programmed death (PD)-1 antibody, in Japanese patients with advanced malignancies, PMID: 33146741
Immune checkpoint inhibitors to treat cutaneous malignancies, PMID: 32461079
RF-Cemiplimab: First Drug Approved for the Treatment of Metastatic or Unresectable Cutaneous Squamous Cell Carcinoma, PMID: 31874704
Correction to: Cemiplimab: A Review in Advanced Cutaneous Squamous Cell Carcinoma, PMID: 32474894
Update of the Management of Cutaneous Squamous-cell Carcinoma, PMID: 32346744
Epidermolysis bullosa: Advances in research and treatment, PMID: 31140655
Simultaneous response of cutaneous and lung squamous cell carcinoma with cemiplimab, PMID: 32614130
Immune Checkpoint Inhibitor-Related Pulmonary Toxicity: Focus on Nivolumab, PMID: 33140115
[Cutaneous squamous cell carcinoma], PMID: 32583034
Posterior Uveitis Associated with Cemiplimab, PMID: 33793370
Actinic Keratosis and Cutaneous Squamous Cell Carcinoma, PMID: 32048593
The Landmark Series: Non-melanoma Skin Cancers, PMID: 31549317
Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial, PMID: 33581821
Bullous pemphigoid associated with cemiplimab therapy in a patient with locally advanced cutaneous squamous cell carcinoma, PMID: 32149176
Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology, PMID: 30917623
Cutaneous squamous cell carcinoma presenting with facial paralysis: good response to Cemiplimab, PMID: 32930485
Multidisciplinary management of locally advanced and metastatic cutaneous squamous cell carcinoma, PMID: 32905333
Population pharmacokinetic characteristics of cemiplimab in patients with advanced malignancies, PMID: 33728546
Cemiplimab is a new option in BCC, PMID: 34035503
A Case of Miller Fisher Syndrome Due to the Use of Cemiplimab, PMID: 32868575
Novel Therapeutics for Recurrent Cervical Cancer: Moving Towards Personalized Therapy, PMID: 31939072
Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial, PMID: 34000246
Cemiplimab removed from reimbursable drugs in France, PMID: 33812142
Cemiplimab: a new option for the treatment of non-small-cell lung cancer, PMID: 34047193
European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment, PMID: 32113942
Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management [Formula: see text], PMID: 32746624
Medical treatment of advanced cutaneous squamous-cell carcinoma, PMID: 31833610
Fixed Dose of Cemiplimab in Patients with Advanced Malignancies Based on Population Pharmacokinetic Analysis, PMID: 33768419
Successful treatment of recurrent advanced cutaneous squamous cell carcinoma with cemiplimab, PMID: 33147669
Immune Checkpoint Inhibitor-associated Uveitis: Review of Treatments and Outcomes, PMID: 32815757
Cemiplimab for Cisplatin Resistant Metastatic Penile Cancer, PMID: 34267641
Cemiplimab-Induced Colitis Causing Hypovolemic Shock: A Case Report and Literature Review., PMID:40520849
Safety, efficacy, and quality of life with cemiplimab treatment among non-small cell lung cancer patients: a systematic review and meta-analysis., PMID:40486556
Integration of immunotherapy and radiotherapy in a therapeutic algorithm for locally advanced squamous cell skin cancer., PMID:40465096
Adjuvant Cemiplimab or Placebo in High-Risk Cutaneous Squamous-Cell Carcinoma., PMID:40454639
Top advances of the year: Developments of immunotherapy in cutaneous squamous cell carcinoma, 2023-2024., PMID:40445861
Cemiplimab in the treatment of metastatic basal cell carcinoma., PMID:40432222
Radiological, Pathological, and Surgical Outcomes with Neoadjuvant Cemiplimab for Stage II-IV Cutaneous Squamous Cell Carcinoma in the Deep Sequencing in Cutaneous Squamous Cell Carcinomas (DISCERN) Trial., PMID:40427224
Updates in Management for Local Regionally Advanced Squamous Cell Carcinoma., PMID:40412889
Successful use of cemiplimab in a high immunologic risk kidney transplant recipient with metastatic squamous cell carcinoma., PMID:40412559
Cetuximab plus 5-fluorouracil in patients with advanced cutaneous squamous cell carcinoma: a retrospective cohort study., PMID:40405694
Association of Sinonasal Symptoms and Disease With Immune Checkpoint Inhibitor Therapy., PMID:40370338
Atypical fibroxanthoma successfully treated with a single dose of cemiplimab., PMID:40357929
From neglect to necessity: the role of innate immunity in cutaneous squamous cell carcinoma therapy., PMID:40352926
Cemiplimab monotherapy as first-line treatment of patients with brain metastases from advanced non-small cell lung cancer with programmed cell death-ligand 1 ≥50., PMID:40323717
Exposed Necrotic Bone in a Head and Neck Cancer Patient: Report of a Diagnostic Challenge., PMID:40310399
PD-L1 Inhibitor Cosibelimab for Cutaneous Squamous Cell Carcinoma: Comprehensive Evaluation of Efficacy, Mechanism, and Clinical Trial Insights., PMID:40299523
Current treatment options for locally advanced and metastatic basal cell carcinoma. A narrative review., PMID:40296667
Pharmacovigilance study on the reporting frequency of atrial fibrillation with immune checkpoint inhibitors: insights from FDA Adverse Event Reporting System., PMID:40290514
Grade 3 and Grade 4 Cutaneous toxicities in patients across multiple solid tumour types receiving checkpoint inhibitor therapy: An observational study. The experience of a single large specialist institution., PMID:40241399
PD-1 Inhibitors for Periocular Squamous Cell Carcinoma With Perineural Spread to the Orbit and Skull Base., PMID:40237556
Acute Infusion Pain Reaction Due to Anti-PD-1 Antibodies for the Treatment of Cutaneous Squamous Cell Carcinoma in Recessive Dystrophic Epidermolysis Bullosa: A Case Report and Review of the Literature., PMID:40225302
Cemiplimab and diabetic ketoacidosis: a case report of a rare endocrinopathy associated with immune checkpoint inhibitors., PMID:40201762
Current Efficacy and Safety and Survival Outcomes of Cemiplimab in Advanced Cutaneous Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis., PMID:40180032
Immune checkpoint inhibitors-induced pancreatitis: a systematic review and real-world pharmacovigilance analysis., PMID:40176908
Immunotherapy followed by cetuximab in locally advanced/metastatic cutaneous squamous cell carcinomas: the I-TACKLE trial., PMID:40154210
Cemiplimab plus peltopepimut-S vaccine in recurrent cervical cancer: A phase 2 clinical trial., PMID:40154184
A Phase I, First-in-Human, Dose-Escalation, Expansion Trial of Cytokine-Encoding Synthetic mRNA Mixture Alone or with Cemiplimab in Advanced Solid Tumors., PMID:40152791
Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study., PMID:40136372
Managing Advanced Basal Cell Carcinoma: A Guide for the Dermatology Clinician., PMID:40135179
Cemiplimab Monotherapy for First-Line Treatment of Patients with Advanced NSCLC With PD-L1 Expression of 50% or Higher: Five-Year Outcomes of EMPOWER-Lung 1., PMID:40118215
Non-Melanoma Skin Cancer Aggravation in Polycythemia Vera: Triggered by Ruxolitinib and Silenced With an IFN/Cemiplimab., PMID:40117636
Combination of Cemiplimab and Radiotherapy in Advanced Squamous Cell Carcinoma., PMID:40117626
Cemiplimab for recurrent locally advanced cutaneous squamous cell carcinoma., PMID:40110840
Effectiveness and Toxicity of Cemiplimab Therapy for Advanced Cutaneous Squamous Cell Skin Cancer in a Community Oncology Practice., PMID:40075670
Efficacy of Combined CD38 and PD1 Inhibition with Isatuximab and Cemiplimab for Relapsed/Refractory NK/T-Cell Lymphoma., PMID:40073374
Eosinophil-induced adverse events induced by treatment with programmed cell death 1/ligand 1 inhibitors: A comprehensive disproportionality analysis of the FDA adverse event reporting system., PMID:40065495
Angiosarcoma: Role of Immunotherapy., PMID:40056281
Immune checkpoint inhibitors for children with xeroderma pigmentosum and advanced cutaneous squamous cell carcinoma: A case presentation and brief review., PMID:40052589
Treatment with programmed-death-1 inhibitors for non-melanoma skin cancer among immunocompromised patients with subgroup analysis of solid organ transplant patients., PMID:40037617
Aggressive cutaneous squamous cell carcinoma in a patient on Janus-kinase inhibitor therapy., PMID:40027434
Immunotherapy for orbital squamous cell carcinoma., PMID:40013614
Case report: Complete response of recurrent locally advanced basal cell carcinoma following addition of vismodegib to neoadjuvant cemiplimab therapy., PMID:39949752
Cosibelimab (Unloxcyt) for cutaneous squamous cell carcinoma., PMID:39946702
Comparative efficacy and safety of first‑line PD‑1/PD‑L1 inhibitors in immunotherapy for non‑small cell lung cancer: A network meta‑analysis., PMID:39916949
Cost-effectiveness of cemiplimab plus chemotherapy vs pembrolizumab plus chemotherapy as first-line treatment for advanced non-small cell lung cancer., PMID:39912814
Pembrolizumab for the Treatment of Locally Advanced Cutaneous Squamous Cell Carcinoma: Patient Selection and Special Considerations., PMID:39912096
Self-Organizing Map-Based Assessment of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors., PMID:39902026
Recent advances in immunotherapy for cervical cancer., PMID:39888529
From basic to clinical and therapeutic insights: news on actinic keratosis and skin squamous cell carcinoma., PMID:39869042
A network comparison on efficacy and safety profiling of PD-1/PD-L1 inhibitors in first-line treatment of advanced non-small cell lung cancer., PMID:39834801