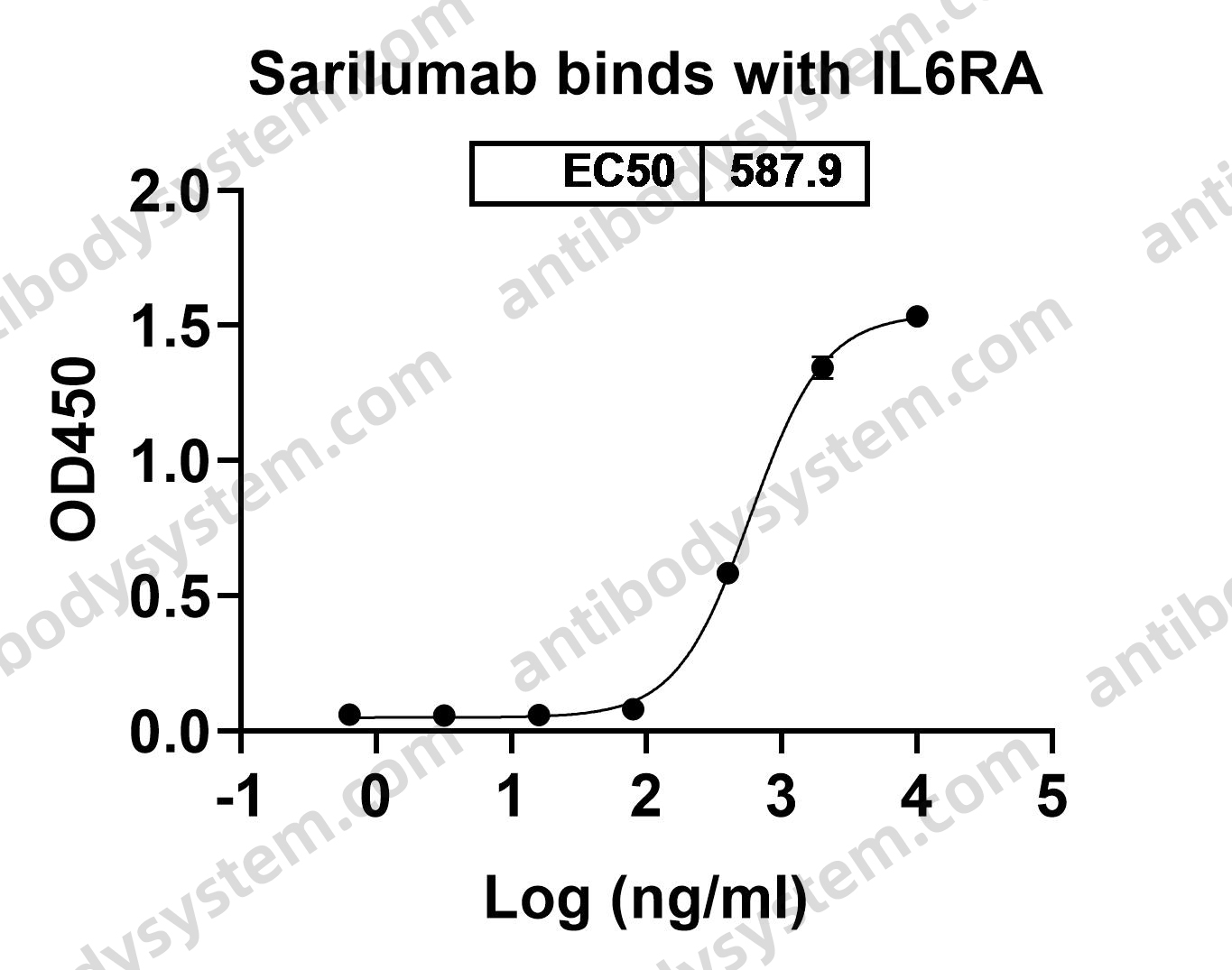
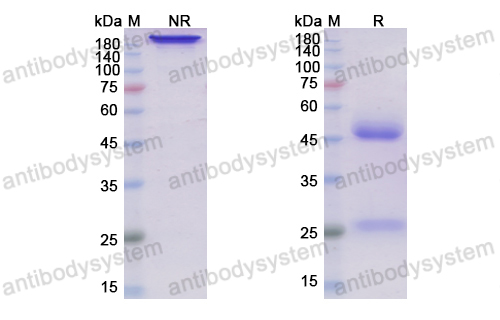
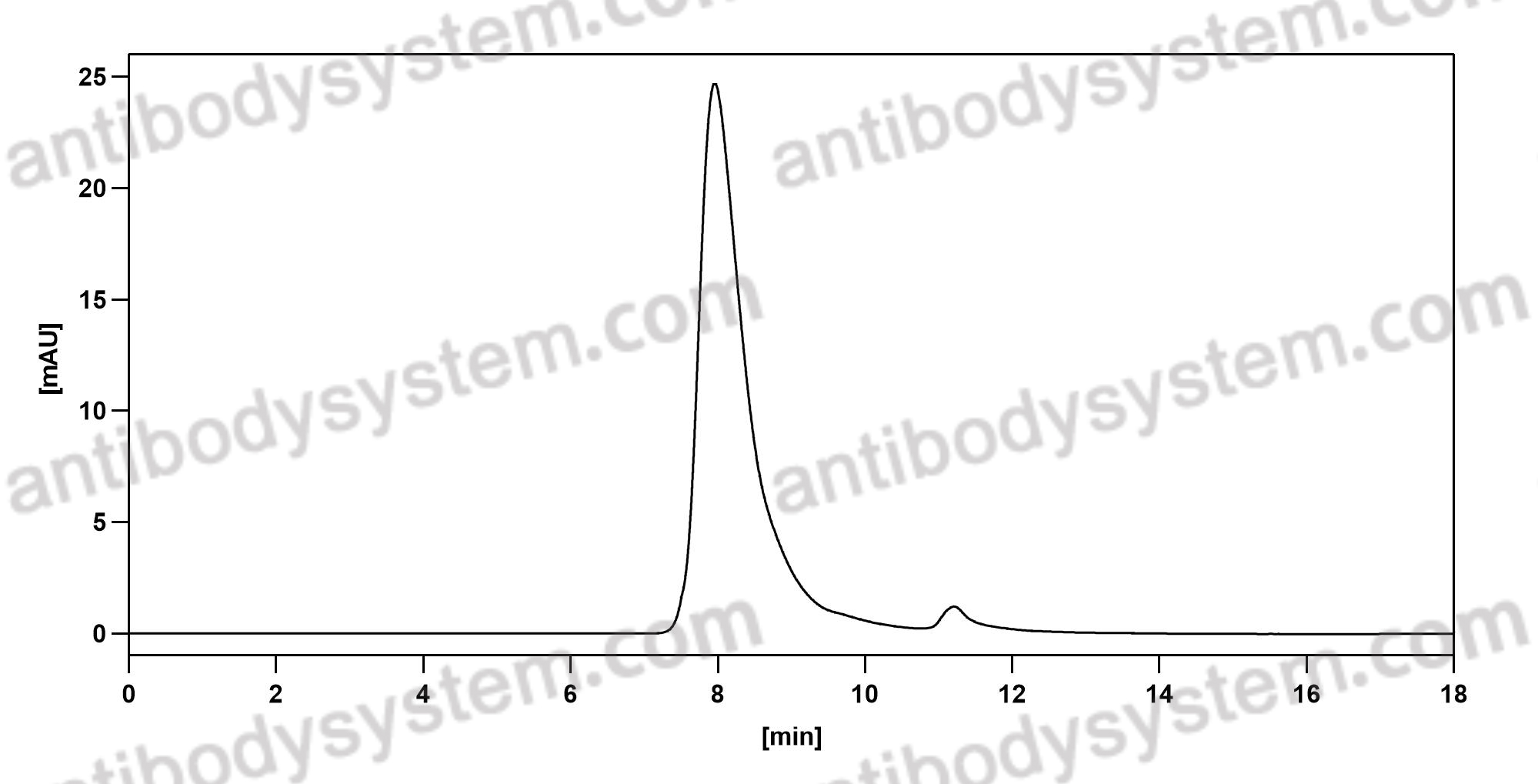
Sarilumab use in severe SARS-CoV-2 pneumonia, PMID: 33043284
Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study, PMID: 32620597
Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial, PMID: 27856432
Sarilumab: A Review in Moderate to Severe Rheumatoid Arthritis, PMID: 29931592
Sarilumab, PMID: 31643297
Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis, PMID: 30590833
Sarilumab, PMID: 29999974
Pharmaco-Immunomodulatory Therapy in COVID-19, PMID: 32696108
Sarilumab: Review of a Second IL-6 Receptor Antagonist Indicated for the Treatment of Rheumatoid Arthritis, PMID: 29482351
A comprehensive review on sarilumab in COVID-19, PMID: 33161757
Sarilumab: patient-reported outcomes in rheumatoid arthritis, PMID: 30154675
Subcutaneous Sarilumab in Patients With Rheumatoid Arthritis who Previously Received Subcutaneous Sarilumab or Intravenous Tocilizumab: An Open-Label Extension of a Randomized Clinical Trial, PMID: 33164349
Sarilumab: First Global Approval, PMID: 28290137
Profile of sarilumab and its potential in the treatment of rheumatoid arthritis, PMID: 28579757
Sarilumab Plus Methotrexate in Patients With Active Rheumatoid Arthritis and Inadequate Response to Methotrexate: Results of a Phase III Study, PMID: 25733246
Differential Binding of Sarilumab and Tocilizumab to IL-6Rα and Effects of Receptor Occupancy on Clinical Parameters, PMID: 33314148
Subcutaneous Sarilumab in hospitalised patients with moderate-severe COVID-19 infection compared to the standard of care (SARCOVID): a structured summary of a study protocol for a randomised controlled trial, PMID: 32907638
Sarilumab versus standard of care for the early treatment of COVID-19 pneumonia in hospitalized patients: SARTRE: a structured summary of a study protocol for a randomised controlled trial, PMID: 32938496
EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update, PMID: 31969328
Pharmacokinetics and Pharmacodynamics of Subcutaneous Sarilumab and Intravenous Tocilizumab Following Single-Dose Administration in Patients With Active Rheumatoid Arthritis on Stable Methotrexate, PMID: 32726514
An Update on Current Therapeutic Drugs Treating COVID-19, PMID: 32395418
Association of High Serum Interleukin-6 Levels With Severe Progression of Rheumatoid Arthritis and Increased Treatment Response Differentiating Sarilumab From Adalimumab or Methotrexate in a Post Hoc Analysis, PMID: 32343882
Il-6 Involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor sarilumab, PMID: 31536783
Pharmacoeconomic Review Report: sarilumab (Kevzara) [Internet], PMID: 30489710
Sarilumab and adalimumab differential effects on bone remodelling and cardiovascular risk biomarkers, and predictions of treatment outcomes, PMID: 32264972
Population Pharmacokinetics of Sarilumab in Patients with Rheumatoid Arthritis, PMID: 31055792
Crisaborole, Dupilumab, and Sarilumab, PMID: 28882252
Room for more IL-6 blockade? Sarilumab for the treatment of rheumatoid arthritis, PMID: 27464017
Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a randomized, placebo-controlled phase III trial in Japan, PMID: 30894208
Evaluation of the efficacy and safety of sarilumab combination therapy in patients with rheumatoid arthritis with inadequate response to conventional disease-modifying antirheumatic drugs or tumour necrosis factor α inhibitors: systematic literature review and network meta-analyses, PMID: 30886733
Potential therapeutic agents against COVID-19: What we know so far, PMID: 32243270
Treatment of SARS-CoV-2: How far have we reached?, PMID: 32336723
IL-6: Relevance for immunopathology of SARS-CoV-2, PMID: 32475759
Efficacy and safety of sarilumab in patients with active rheumatoid arthritis, PMID: 29492111
Clinical Review Report: sarilumab (Kevzara) [Internet], PMID: 30489709
A living WHO guideline on drugs for covid-19, PMID: 32887691
Long-term safety of sarilumab in rheumatoid arthritis: an integrated analysis with up to 7 years' follow-up, PMID: 31312844
EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update, PMID: 28264816
Efficacy and safety of sarilumab in combination with csDMARDs or as monotherapy in subpopulations of patients with moderately to severely active rheumatoid arthritis in three phase III randomized, controlled studies, PMID: 32522251
Current status of potential therapeutic candidates for the COVID-19 crisis, PMID: 32334062
Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy, PMID: 32784217
Effects of Sarilumab on Rheumatoid Arthritis as Reported by Patients Using the Rheumatoid Arthritis Impact of Disease Scale, PMID: 30877216
Immunogenicity of Sarilumab Monotherapy in Patients with Rheumatoid Arthritis Who Were Inadequate Responders or Intolerant to Disease-Modifying Antirheumatic Drugs, PMID: 31090044
Treatment Considerations for COVID-19: A Critical Review of the Evidence (or Lack Thereof), PMID: 32561148
Sarilumab for Previously-Treated Moderate or Severe Rheumatoid Arthritis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal, PMID: 29882210
A review of sarilumab for the treatment of rheumatoid arthritis, PMID: 29043871
Hyperinflammation and the utility of immunomodulatory medications in children with COVID-19, PMID: 32792288
Sarilumab monotherapy compared with adalimumab monotherapy for the treatment of moderately to severely active rheumatoid arthritis: an analysis of incremental cost per effectively treated patient, PMID: 30787625
Potential strategies for combating COVID-19, PMID: 32778950
Assessing the Value of Sarilumab Monotherapy for Adults with Moderately to Severely Active Rheumatoid Arthritis: A Cost-Effectiveness Analysis, PMID: 30589626
Treatment of indolent systemic mastocytosis with sarilumab is not supported in a randomized trial., PMID:40529483
Sarilumab in the treatment of rheumatoid arthritis: future perspectives., PMID:40506803
New treatments for systemic mastocytosis in 2025., PMID:40471046
Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis., PMID:40393718
Tocilizumab, sarilumab and anakinra in critically ill patients with COVID-19: a randomised, controlled, open-label, adaptive platform trial., PMID:40360262
Abatacept, Golimumab, and Sarilumab as Selected Bio-Originator Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action in Their Current Use in Treatment., PMID:40142915
Cost-effectiveness analysis of subcutaneous biosimilar tocilizumab in patients with rheumatoid arthritis in Spain., PMID:40113553
Population Pharmacokinetics and Exposure-Response Analyses of Sarilumab in Patients with Polymyalgia Rheumatica., PMID:40105153
Successful Salvage Therapy of Late-onset Arterial Disorders due to Recurrent Vasospasms Following Free Flap Transfer under the IL-6 and TNF-α Signaling-downregulated Environment: A Case Report., PMID:40104165
Anti-Rheumatic potential of biological DMARDS and protagonistic role of bio-markers in early detection and management of rheumatoid arthritis., PMID:40091354
Advances in the treatment of polymyalgia rheumatica., PMID:40071422
Comparative effectiveness of subcutaneous sarilumab 200 mg biweekly, subcutaneous Tocilizumab 162 mg biweekly, and intravenous Tocilizumab 8 mg/kg every 4 weeks in patients with rheumatoid arthritis: a prospective cohort study., PMID:40055759
Interleukin 6 inhibition in refractory antisynthetase syndrome: case-based literature review., PMID:40053413
Long-term safety and efficacy of anti-GM-CSF otilimab in patients with rheumatoid arthritis: long-term extension of three phase 3 randomised trials (contRAst X)., PMID:40044198
Nontraumatic terminal ileal perforation in a patient with resistant palindromic rheumatism treated with sarilumab: A case report., PMID:40024808
Effectiveness of baricitinib versus sarilumab on disease activity in patients with RA: a propensity score matching study., PMID:39959132
[Rheumatology: what's new in 2024]., PMID:39881648
Terminal complement complex deposition on chondrocytes promotes premature senescence in age- and trauma-related osteoarthritis., PMID:39877352
Safety and Efficacy of Baricitinib, Upadacitinib, and Sarilumab Implementation in Moderately-to-Severely Active Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Current Evidence., PMID:39867028
IL-6R Inhibitors and Gastrointestinal Perforations: A Pharmacovigilance Study and a Predicting Nomogram., PMID:39767766
Successful Remission of Refractory Rheumatoid Pleural Effusion and Arthritis Using Sarilumab: A Case Report., PMID:39744302
Long-term efficacy of sarilumab on the progression of interstitial lung disease in rheumatoid arthritis: the KEIO-RA cohort and literature review., PMID:39711360
The pattern of anti-IL-6 versus non-anti-IL-6 biologic disease modifying anti-rheumatic drugs use in patients with rheumatoid arthritis in Wales, UK: a real-world study using electronic health records., PMID:39698233
Cost-effectiveness analysis of subcutaneous biosimilar tocilizumab in patients with rheumatoid arthritis in Spain., PMID:39675937
Rates of glucocorticoid taper in the management of polymyalgia rheumatica: the science behind the "art"., PMID:39641835
A Glimpse for the subsistence from pandemic SARS-CoV-2 infection., PMID:39603070
Leveraging large-scale multi-omics evidences to identify therapeutic targets from genome-wide association studies., PMID:39563277
Evaluating Meaningful Changes in Patient-Reported Outcome Measurement Information System-Fatigue Scores from Three Phase 3 Clinical Trials of Sarilumab for Patients With Rheumatoid Arthritis., PMID:39545383
Traditional and Emerging Strategies for Managing Polymyalgia Rheumatica: Insights into New Treatments., PMID:39518631
Biological and Therapeutic Role of Interleukin-6 in Non-Infectious Uveitis: A Narrative Review., PMID:39404707
Evaluating anchor variables and variation in meaningful score differences for PROMIS® Pediatric measures in children and adolescents living with a rheumatic disease., PMID:39400691
Real-world Effectiveness of Sarilumab in RA: Results from the Open-label, Prospective, Single-arm Observational PROFILE Study., PMID:39287897
A case of VEXAS with microcytic anemia: don't be mislead by an associated condition!, PMID:39279630
Usefulness of Sarilumab in Patients with Rheumatoid Arthritis after Regression of Lymphoproliferative Disorders., PMID:39262417
Investigating the impact of Tocilizumab, Sarilumab, and Anakinra on clinical outcomes in COVID-19: A systematic review and meta-analysis., PMID:39221116
Pain Interference in Juvenile Idiopathic Arthritis., PMID:39218453
[Polymyalgia rheumatica: What's new?]., PMID:39146754
The pipeline of immunomodulatory therapies in polymyalgia rheumatica and giant cell arteritis: A systematic review of clinical trials., PMID:39122202
Use of interleukin-6 receptor antibodies in the second and third trimester of pregnancy: a retrospective cohort study., PMID:39116898
Newer Therapies in Rheumatology., PMID:39084836
The effects of sarilumab as monotherapy and in combination with non-methotrexate disease-modifying anti-rheumatic drugs on unacceptable pain in patients with rheumatoid arthritis: A post-hoc analysis of the HARUKA phase 3 study., PMID:39073577
Efficacy and safety of sarilumab in patients with rheumatoid arthritis stratified by age (<65 and ≥65 years): A post hoc analysis of Japanese Phase 3 clinical trials., PMID:39073574
Assessment of adverse events related to anti-interleukin-6 receptor monoclonal antibodies using the FDA adverse event reporting system: a real-world pharmacovigilance study., PMID:39049740
Efficacy of sarilumab and dexamethasone co-administration for lowering multiple blood biomarkers in the treatment of cytokine release syndrome in hospitalized COVID-19 patients., PMID:39028068
Investigational agents for polymyalgia rheumatica treatment: assessing the critical needs for future development., PMID:38879822
Safety of sarilumab in a Japanese population with rheumatoid arthritis by age group: Data from an interim analysis of a postmarketing surveillance study., PMID:38804962
Effect of IL-6R blockade on plasma lipids and clinical outcomes among hospitalized patients with COVID-19 infection., PMID:38795859
Late Tuberculosis Reactivation After Severe Covid-19., PMID:38715882
Sarilumab (Kevzara) for polymyalgia rheumatica., PMID:38696312