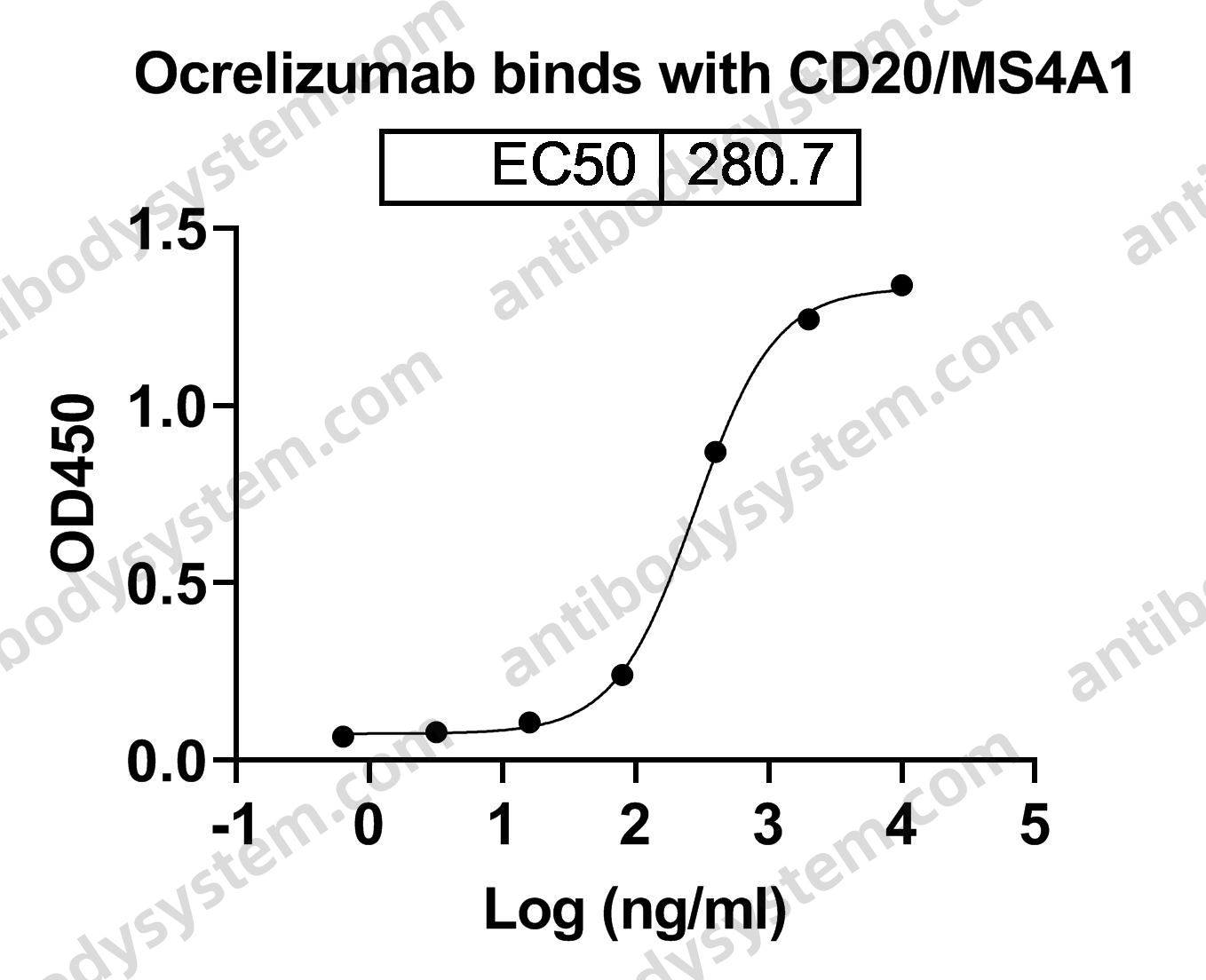
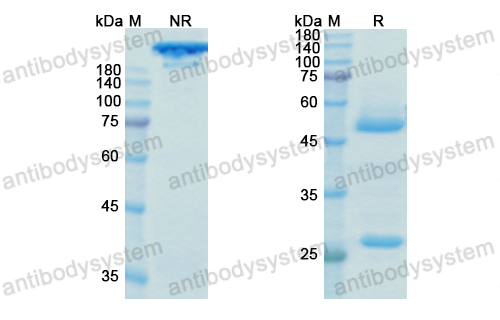
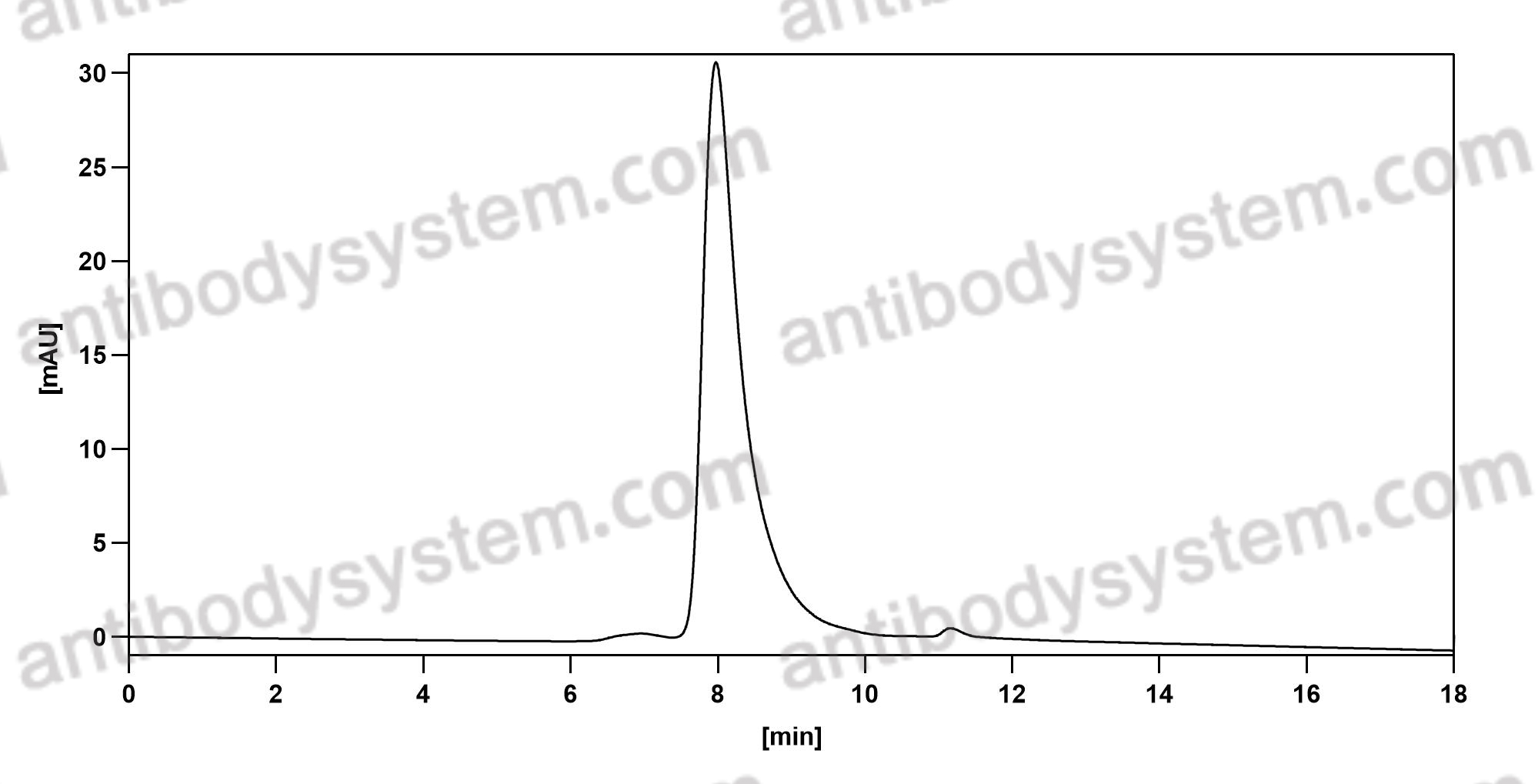
Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis, PMID: 28002679
Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis, PMID: 28002688
Ocrelizumab: A Review in Multiple Sclerosis, PMID: 30171504
Ocrelizumab and Other CD20 + B-Cell-Depleting Therapies in Multiple Sclerosis, PMID: 28695471
Ocrelizumab: its efficacy and safety in multiple sclerosis, PMID: 29897610
[Ocrelizumab for treatment of multiple sclerosis], PMID: 32524163
Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study, PMID: 32727835
Ocrelizumab, PMID: 31613530
Ocrelizumab: a new milestone in multiple sclerosis therapy, PMID: 29774057
Ocrelizumab for the treatment of multiple sclerosis, PMID: 30570368
Anti-CD20 Agents for Multiple Sclerosis: Spotlight on Ocrelizumab and Ofatumumab, PMID: 33092190
COVID-19 in ocrelizumab-treated people with multiple sclerosis, PMID: 33482590
Ocrelizumab, PMID: 31643475
Ocrelizumab for multiple sclerosis, PMID: 30116083
Onset of clinical and MRI efficacy of ocrelizumab in relapsing multiple sclerosis, PMID: 31484710
Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis, PMID: 30677733
Ocrelizumab, PMID: 29999962
Adverse event profile differences between rituximab and ocrelizumab: Findings from the FDA Adverse Event Reporting Database, PMID: 32820687
Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies, PMID: 30844611
Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis, PMID: 30820738
COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases, PMID: 32671831
Ocrelizumab for Treating Patients with Primary Progressive Multiple Sclerosis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal, PMID: 32048205
The ocrelizumab phase II extension trial suggests the potential to improve the risk: Benefit balance in multiple sclerosis, PMID: 32645640
Ocrelizumab-Induced Severe Colitis, PMID: 33354373
Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial, PMID: 22047971
Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension, PMID: 32690791
Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial, PMID: 33129442
Dermatographism associated with ocrelizumab, PMID: 32979732
COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role?, PMID: 32315980
COVID-19 in persons with multiple sclerosis treated with ocrelizumab - A pharmacovigilance case series, PMID: 32570202
Ocrelizumab initiation in patients with MS: A multicenter observational study, PMID: 32273482
Long-term ocrelizumab in progressive multiple sclerosis, PMID: 33129443
Ocrelizumab Treatment in Patients with Primary Progressive Multiple Sclerosis: Short-term Safety Results from a Compassionate Use Programme in Germany, PMID: 32920498
Multiple Sclerosis Disease-Modifying Therapy and the COVID-19 Pandemic: Implications on the Risk of Infection and Future Vaccination, PMID: 32780300
Ocrelizumab for multiple sclerosis, PMID: 30008443
Formulary Drug Review: Ocrelizumab, PMID: 29276296
Ocrelizumab: A New B-cell Therapy for Relapsing Remitting and Primary Progressive Multiple Sclerosis, PMID: 29232960
Ocrelizumab for the treatment of relapsing-remitting multiple sclerosis, PMID: 27552111
Ocrelizumab for primary progressive multiple sclerosis, PMID: 31279744
Real-World Results of Ocrelizumab Treatment for Primary Progressive Multiple Sclerosis, PMID: 32607256
Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness, PMID: 33370649
Safety profile of ocrelizumab for the treatment of multiple sclerosis: a systematic review, PMID: 32799563
Endocarditis following ocrelizumab in relapsing-remitting MS, PMID: 32014890
Ocrelizumab: a B-cell depleting therapy for multiple sclerosis, PMID: 28658986
Ocrelizumab in relapsing and primary progressive multiple sclerosis: Pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO, PMID: 33202059
Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population, PMID: 33369288
Switching from natalizumab to ocrelizumab in patients with multiple sclerosis, PMID: 32552363
Pharmacoeconomic Review Report: Ocrelizumab (Ocrevus): (Hoffmann-La Roche Limited) [Internet], PMID: 30462439
Clinical Review Report: Ocrelizumab (Ocrevus): (Hoffmann-La Roche Limited) [Internet], PMID: 30457779
Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine?, PMID: 32619884
Clinical Characteristics and Chronic Immunotherapy in Idiopathic Recurrent Neuroretinitis and Idiopathic Recurrent Papillitis: Describing a Potential New Phenotype., PMID:40528302
Native structure of the monoclonal therapeutic CD20 antibody ocrelizumab., PMID:40513927
Role of Ocrelizumab in modulating gene and microRNA expression in multiple sclerosis., PMID:40510879
Progression independent of relapsing biology in multiple sclerosis: a real-word study., PMID:40510203
Effectiveness of anti-CD20 therapies following natalizumab discontinuation: insights from a cohort study., PMID:40505538
Ocrelizumab as first-line therapy in highly active relapsing-remitting multiple sclerosis., PMID:40505352
Effect of Cumulative Exposure to Ocrelizumab on Memory B-Cell Repopulation Dynamics in Multiple Sclerosis., PMID:40497506
What Is the Evidence on Immunomodulators and Immunosuppressants for Progressive Multiple Sclerosis? - A Cochrane Review Summary with Commentary., PMID:40485316
De-escalation of disease modifying therapies: A retrospective, observational single center study., PMID:40480031
The Italian Multiple Sclerosis Register Experience With Cladribine: Impact on Relapses, PIRA, and Treatment Sequencing Strategies Evaluation., PMID:40472290
CONFIDENCE treatment success: long-term real-world effectiveness and safety of ocrelizumab in Germany., PMID:40470489
A disproportionality analysis of nervous system adverse events associated with disease-modifying therapies in multiple sclerosis: insights from the FDA adverse event reporting system (FAERS)., PMID:40465056
Persistence to Ocrelizumab Compared with Other Disease-Modifying Therapies for Multiple Sclerosis: Results from the German NeuroTransData Registry., PMID:40455371
Comparative effectiveness of natalizumab and anti-CD20 monoclonal antibodies in relapsing-remitting multiple sclerosis: a real-world propensity-score matched study., PMID:40451284
Real-world evidence from Türkiye on cancer risk and treatment exposure in multiple sclerosis: A histopathology-verified, registry-based study., PMID:40449191
Ocrelizumab-Induced Hemophagocytic Lymphohistiocytosis: A Case Report., PMID:40438805
Ocrelizumab for pediatric relapsing multiple sclerosis., PMID:40435665
Vaccine-Induced Humoral and Cellular Response to SARS-CoV-2 in Multiple Sclerosis Patients on Ocrelizumab., PMID:40432100
Real-world observational study of infections in people treated with ocrelizumab for multiple sclerosis., PMID:40402264
Interventions promoting remyelination in multiple sclerosis: a systematic review of clinical trials., PMID:40393003
Pediatric Multiple Sclerosis: A Systematic Exploration of Effectiveness in Current and Emerging Therapeutics., PMID:40381455
Seronegative spondyloarthritis as a complication of anti-CD20 monoclonal antibody treatment in multiple sclerosis., PMID:40367683
Societal Versus Healthcare Perspectives on the Cost Effectiveness of Ocrelizumab for Treatment of Primary Progressive Multiple Sclerosis in Aotearoa New Zealand., PMID:40332665
Multiple Sclerosis Disease-Modifying Treatment Algorithms: 2025 Positioning of the Portuguese Multiple Sclerosis Study Group., PMID:40326993
Comprehensive analysis of B cell repopulation in ocrelizumab-treated patients with multiple sclerosis by mass cytometry and proteomics., PMID:40322080
Case Report: Neurofilament light chain in the follow up of progressive multifocal leukoencephalopathy in a patient with multiple sclerosis treated with ocrelizumab., PMID:40308763
Cost-effectiveness of ofatumumab for the treatment of relapsing forms of multiple sclerosis in the United Arab Emirates., PMID:40303316
Severe Late-Onset Neutropenia in a Pregnant Patient with Multiple Sclerosis after Ocrelizumab., PMID:40292041
Switching to ublituximab from prior anti-CD20 monoclonal antibody therapy: a case report series., PMID:40255393
Effectiveness of Autologous Hematopoietic Stem Cell Transplantation versus Alemtuzumab and Ocrelizumab in Relapsing Multiple Sclerosis: A Single Center Cohort Study., PMID:40251896
Subcutaneous Ocrelizumab in Patients With Multiple Sclerosis: Results of the Phase 3 OCARINA II Study., PMID:40245351
Short- and long-term effects of early versus delayed treatment with ocrelizumab on cerebellar volume loss in patients with RMS and PPMS., PMID:40237070
Hypogammaglobulinemia in patients with multiple sclerosis receiving disease modifying therapies: disproportionality analysis using the EudraVigilance database., PMID:40235349
Risk of Serious Infections in Patients Treated With Biologic or Targeted-synthetic Disease Modifying Antirheumatic Drugs in Qatar., PMID:40230031
Serological screening and Vaccine Update in a cohort of Multiple Sclerosis Patients as a strategy to prevent infection reactivation during immunosuppressant therapy., PMID:40220724
Efficacy of ocrelizumab versus rituximab in patients with relapsing-remitting multiple sclerosis., PMID:40216701
Effects of Anti-CD20 Antibody Therapy on Immune Cell Dynamics in Relapsing-Remitting Multiple Sclerosis., PMID:40214505
Teaching NeuroImage: Severe Enterovirus Encephalitis as a Complication of Ocrelizumab Treatment for Multiple Sclerosis., PMID:40209134
Treatment-resistant inflammatory demyelinating pseudotumor with Marburg-like features: A narrative review-based treatment approach., PMID:40200967
Plasmapheresis for Facilitating Readministration of Rituximab After Paradoxical Exacerbation in Pemphigus Vulgaris: A Case Report., PMID:40196047
Recall vaccination increases detectable B-cell reactivity in persons with multiple sclerosis treated with ocrelizumab., PMID:40186086
Clinical and radiological activity after extended interval and standard interval dosing of ocrelizumab in multiple sclerosis: A systematic review and meta-analysis., PMID:40183837
The Evolution of Anti-CD20 Treatment for Multiple Sclerosis: Optimization of Antibody Characteristics and Function., PMID:40180777
Suboptimal B-cell depletion is associated with progression independent of relapse activity in multiple sclerosis patients treated with ocrelizumab., PMID:40177950
Pyoderma gangrenosum in a patient with multiple sclerosis under natalizumab treatment: a case report., PMID:40175973
Recommendations for essential medicines for multiple sclerosis in low-resource settings., PMID:40167177
Home-sampling of B cells using quantitative dried blood spots to enable tailored therapeutic re-dosing of anti-CD20 therapies., PMID:40167038
Therapeutic Advances in Pediatric Multiple Sclerosis., PMID:40150542
No evidence of fluctuations in daily step count between infusions in people with multiple sclerosis treated with anti-CD20 monoclonal antibodies., PMID:40144903