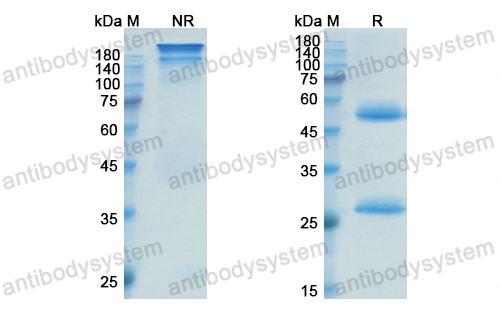
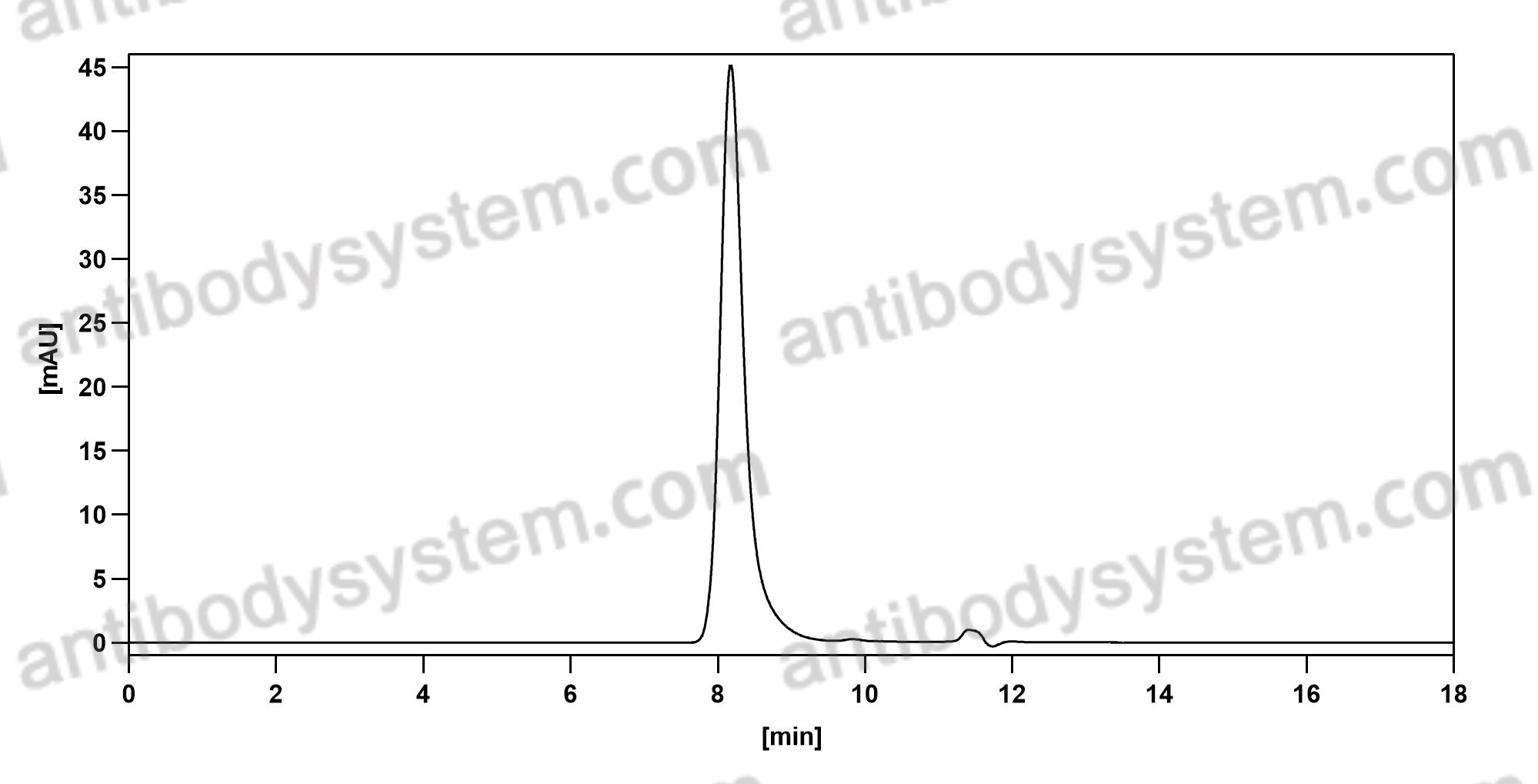
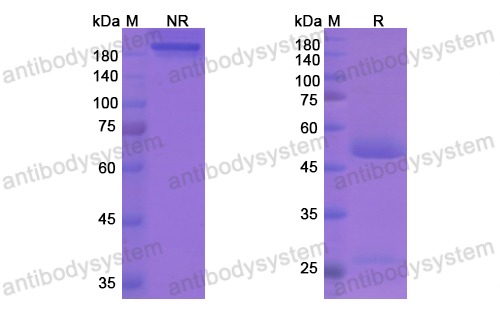
Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia, PMID: 27292104
Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study, PMID: 29352703
Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study, PMID: 30920645
Inotuzumab Ozogamicin: A Review in Relapsed/Refractory B-Cell Acute Lymphoblastic Leukaemia, PMID: 30090971
Salvage Chemoimmunotherapy With Inotuzumab Ozogamicin Combined With Mini-Hyper-CVD for Patients With Relapsed or Refractory Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Phase 2 Clinical Trial, PMID: 28859185
Inotuzumab ozogamicin for acute lymphoblastic leukaemia, PMID: 31427847
Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage, PMID: 30307611
Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia, PMID: 30267011
Role of blinatumomab, inotuzumab, and CAR T-cells: Which to choose and how to sequence for patients with relapsed disease, PMID: 33256906
Inotuzumab Ozogamicin, PMID: 31643242
Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia: outcomes by disease burden, PMID: 32769965
Inotuzumab Ozogamicin: First Global Approval, PMID: 28819740
Inotuzumab Ozogamicin, PMID: 29999985
Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: A propensity score analysis, PMID: 30985931
Clinical utilization of blinatumomab and inotuzumab immunotherapy in children with relapsed or refractory B-acute lymphoblastic leukemia, PMID: 33098744
Inotuzumab ozogamicin in the treatment of relapsed/refractory acute B cell lymphoblastic leukemia, PMID: 29713210
Efficacy of inotuzumab ozogamicin in patients with Philadelphia chromosome-positive relapsed/refractory acute lymphoblastic leukemia, PMID: 33231879
Inotuzumab: from preclinical development to success in B-cell acute lymphoblastic leukemia, PMID: 30622147
Inotuzumab ozogamicin in clinical development for acute lymphoblastic leukemia and non-Hodgkin lymphoma, PMID: 31011424
Impact of minimal residual disease status in patients with relapsed/refractory acute lymphoblastic leukemia treated with inotuzumab ozogamicin in the phase III INO-VATE trial, PMID: 31790983
The Clinical and Cost Effectiveness of Inotuzumab Ozogamicin for the Treatment of Adult Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia: An Evidence Review Group Evaluation of a NICE Single Technology Appraisal, PMID: 30887470
Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study, PMID: 28687420
Hepatic veno-occlusive disease in allogeneic stem cell transplant recipients with prior exposure to gemtuzumab ozogamicin or inotuzumab ozogamicin, PMID: 32988266
A phase 1 study of inotuzumab ozogamicin in pediatric relapsed/refractory acute lymphoblastic leukemia (ITCC-059 study), PMID: 33067614
Outcomes of Allogeneic Stem Cell Transplantation after Inotuzumab Ozogamicin Treatment for Relapsed or Refractory Acute Lymphoblastic Leukemia, PMID: 31039409
Immunotherapy in pediatric acute lymphoblastic leukemia, PMID: 31811553
Inotuzumab ozogamicin in acute lymphoblastic leukaemia, PMID: 30956118
Inotuzumab ozogamicin: a CD22 mAb-drug conjugate for adult relapsed or refractory B-cell precursor acute lymphoblastic leukemia, PMID: 30087554
Inotuzumab ozogamicin for the treatment of acute lymphoblastic leukemia, PMID: 29092647
Inotuzumab ozogamicin in relapsed B-cell acute lymphoblastic leukemia, PMID: 28152223
Adult Acute Lymphoblastic Leukemia, PMID: 27814839
Inotuzumab ozogamicin in the management of acute lymphoblastic leukaemia, PMID: 26654587
Inotuzumab ozogamicin in the treatment of acute lymphoblastic leukemia, PMID: 24389139
Treatment of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia, PMID: 30675645
Recent advances in the treatment of acute lymphoblastic leukemia, PMID: 31092071
Blinatumomab or Inotuzumab Ozogamicin as Bridge to Allogeneic Stem Cell Transplantation for Relapsed or Refractory B-lineage Acute Lymphoblastic Leukemia: A Retrospective Single-Center Analysis, PMID: 32646833
Inotuzumab ozogamicin (Besponsa)--an antibody-drug conjugate for ALL, PMID: 29913470
Role of inotuzumab ozogamicin in the treatment of relapsed/refractory acute lymphoblastic leukemia, PMID: 26780449
Inotuzumab ozogamicin in B-cell acute lymphoblastic leukemias and non-Hodgkin's lymphomas, PMID: 25775418
Inotuzumab ozogamicin in the treatment of B-cell acute lymphoblastic leukemia, PMID: 22500551
Precision medicine in acute lymphoblastic leukemia, PMID: 33074527
Inotuzumab Ozogamicin in Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia, PMID: 31186988
Inotuzumab ozogamicin as novel therapy in lymphomas, PMID: 20528256
CD22 Expression in B-Cell Acute Lymphoblastic Leukemia: Biological Significance and Implications for Inotuzumab Therapy in Adults, PMID: 32012891
Inotuzumab ozogamicin, a nonchemotherapy option for relapsed acute lymphoblastic leukemia, PMID: 31188803
Inotuzumab ozogamicin for the treatment of acute lymphoblastic leukemia, PMID: 33815734
Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies, PMID: 25304309
Inotuzumab ozogamicin for the treatment of patients with acute lymphocytic leukemia, PMID: 29517084
Inotuzumab ozogamicin compassionate use for French paediatric patients with relapsed or refractory CD22-positive B-cell acute lymphoblastic leukaemia, PMID: 32424837
Evolving therapy of adult acute lymphoblastic leukemia: state-of-the-art treatment and future directions, PMID: 32503572
Outcome of second allogeneic hematopoietic cell transplantation in adult patients with relapsed B-cell acute lymphoblastic leukemia in the era of new immunotherapeutic agents., PMID:40527981
Pharmacovigilance insights into antibody-drug conjugates: a multi-database analysis of adverse events in leukemia treatment., PMID:40522479
Comparing Outcomes of First-Line Intensive Chemotherapeutic Regimens in Adult Patients With Acute Lymphoblastic Leukemia at a Tertiary Center., PMID:40500615
Treatment of Older Adults with Newly Diagnosed Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia., PMID:40494331
Acute Lymphoid Leukemia: Is Transplant Indicated for Philadelphia Chromosome Positive ALL?, PMID:40488828
Advances and challenges in leukemia treatment: A focus on monoclonal antibodies and emerging therapies., PMID:40486885
Recent advances in monoclonal antibody development for treatment of B-cell acute lymphoblastic leukemia., PMID:40455243
Revumenib for Relapsed or Refractory Acute Leukemia With a KMT2A Translocation., PMID:40437770
Differentiation-dependent EBF1 Activity Determines CD22 Transcription and Leukemia Sensitivity to Inotuzumab Ozogamicin., PMID:40435412
Identifying people with acute lymphoblastic leukemia who are most suitable for treatment with inotuzumab ozogamicin: a plain language summary., PMID:40394810
Inotuzumab therapy resulted in molecular remission of relapsed/resistant B-cell acute lymphoblastic leukemia with BCR::ABL1 Lys404Glu mutation., PMID:40377672
Adult Acute Lymphoblastic Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring., PMID:40377367
Safety evaluation of inotuzumab ozogamicin: a pharmacovigilance study based on the FAERS database., PMID:40359557
Treatment of Older Patients With ALL., PMID:40354595
Beyond Cellular Therapies: The Expanding Role of Antibody-Driven Immunotherapy in Pediatric Acute Lymphoblastic Leukemia., PMID:40324358
The evolving therapeutic revolution in adult acute lymphoblastic leukemia., PMID:40323723
Management of Adult Acute Lymphoblastic Leukemia: A Review., PMID:40310617
Pre-transplant blinatumomab and/or inotuzumab ozogamicin therapy for relapsed/refractory acute lymphoblastic and B/myeloid mixed phenotype acute leukemia in adults., PMID:40306011
Hepatic sinusoidal congestion associated with inotuzumab therapy in patients with B-cell acute lymphoblastic leukemia, a proposal for a new clinical entity: Calicheamicin syndrome., PMID:40296727
Bispecific antibodies in immunotherapy for acute leukemia: latest updates from the 66th annual meeting of the American society of hematology, 2024., PMID:40270605
Atypical features of hepatic veno-occlusive disease/sinusoidal obstruction syndrome after inotuzumab ozogamicin in adult patients with acute lymphoblastic leukemia., PMID:40261550
CD22-targeted chimeric antigen receptor-modified T cells for children and adults with relapse of B-cell acute lymphoblastic leukemia after CD19-directed immunotherapy., PMID:40246579
Differences in response to immunotherapy drugs including blinatumomab and inotuzumab ozogamicin in B-ALL patients., PMID:40232601
Inferior Outcomes in Acute Lymphoblastic Leukemia With Translocation (14;18)., PMID:40222876
Real-world outcomes for young adult patients receiving CD19 CAR T-cell therapy., PMID:40127395
INO-CD22: A multicenter, real-world study of inotuzumab ozogamicin safety and effectiveness in adult patients with relapsed/refractory acute lymphoblastic leukemia., PMID:40120068
CIBMTR Registry Data on Inotuzumab Ozogamicin Treatment in Patients with ALL Who Proceeded to Hematopoietic Stem Cell Transplant-A Podcast., PMID:40111710
Inotuzumab Ozogamicin as a Bridge to Stem Cell Transplantation in Relapsed Pediatric BCP-ALL After Tisagenlecleucel: A Case Series., PMID:40085546
Antibody-Based and Other Novel Agents in Adult B-Cell Acute Lymphoblastic Leukemia., PMID:40075627
Therapeutic Strategies for Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia in Adult Patients: Optimizing the Use of Monoclonal Antibodies., PMID:40045912
Gastrointestinal toxicity of antibody-drug conjugates: a pharmacovigilance study using the FAERS database., PMID:40033454
Five-Year Real-World Safety of Inotuzumab Ozogamicin Before Hematopoietic Stem Cell Transplantation in B-Cell Precursor Acute Lymphoblastic Leukemia., PMID:39989181
Therapeutic drug monitoring in acute lymphoblastic leukemia-a deep dive into pharmacokinetics, -dynamics, and -genetics of antileukemic drugs., PMID:39949259
Inotuzumab Ozogamicin as First-Line Therapy in Acute Lymphoblastic Leukemia., PMID:39909815
Tumor-specific cytosol-penetrating antibodies for antigen- and TME-dependent intracellular cargo delivery., PMID:39895690
Efficacy and safety of currently approved and lower starting doses of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukemia: a phase IV study., PMID:39882642
CD58 expression does not impact response to inotuzumab ozogamicin in patients with B-cell acute lymphoblastic leukemia., PMID:39866945
Utility of ursodiol prophylaxis against sinusoidal obstruction syndrome (SOS)/ veno-occlusive disease (VOD) in acute leukemia patients receiving gemtuzumab-ozogamicin (GO) or inotuzumab-ozogamicin (InO)., PMID:39819278
Role of Allogeneic Hematopoietic Stem Cell Transplantation for Philadelphia Chromosome-Positive B-Cell Acute Lymphoblastic Leukemia in the Contemporary Era., PMID:39796731
DNTT-mediated DNA damage response drives inotuzumab ozogamicin resistance in B-cell acute lymphoblastic leukemia., PMID:39791601
Frontline immunotherapeutic combination strategies in adult B-cell acute lymphoblastic leukemia: reducing chemotherapy intensity and toxicity and harnessing efficacy., PMID:39791458
Characterizing the ideal patient for treatment with inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia: a systematic literature review., PMID:39778191
Hyper-CVAD and Sequential Blinatumomab Without and With Inotuzumab in Young Adults With Newly Diagnosed Philadelphia Chromosome-Negative B-Cell Acute Lymphoblastic Leukemia., PMID:39757533
Philadelphia Chromosome as a Clinically Favorable Prognostic Factor of B-cell Acute Lymphoblastic Leukemia/Lymphoblastic Lymphoma in Transplant-Ineligible Elderly Patients in the Era of Molecular-Targeted Therapy., PMID:39703243
Contributions of Pediatric Hematology/Oncology to the Diagnosis, Treatment, and Cure of Acute Lymphoblastic Leukemia-Part 2b (Numbers 16 to 20)., PMID:39652786
Inotuzumab ozogamicin for relapse prevention in a boy with Down syndrome and relapsed acute lymphoblastic leukemia., PMID:39648271
Antibody-Drug Conjugates: The Toxicities and Adverse Effects That Emergency Physicians Must Know., PMID:39641680
Blinatumomab for the treatment of acute lymphoblastic leukemia in a real-world setting: clinical vignettes., PMID:39611241
Exploring the role of PARP1 inhibition in enhancing antibody-drug conjugate therapy for acute leukemias: insights from DNA damage response pathway interactions., PMID:39587643