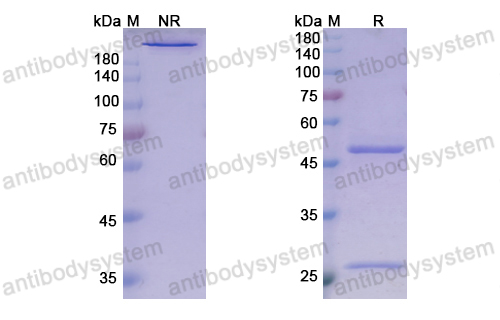
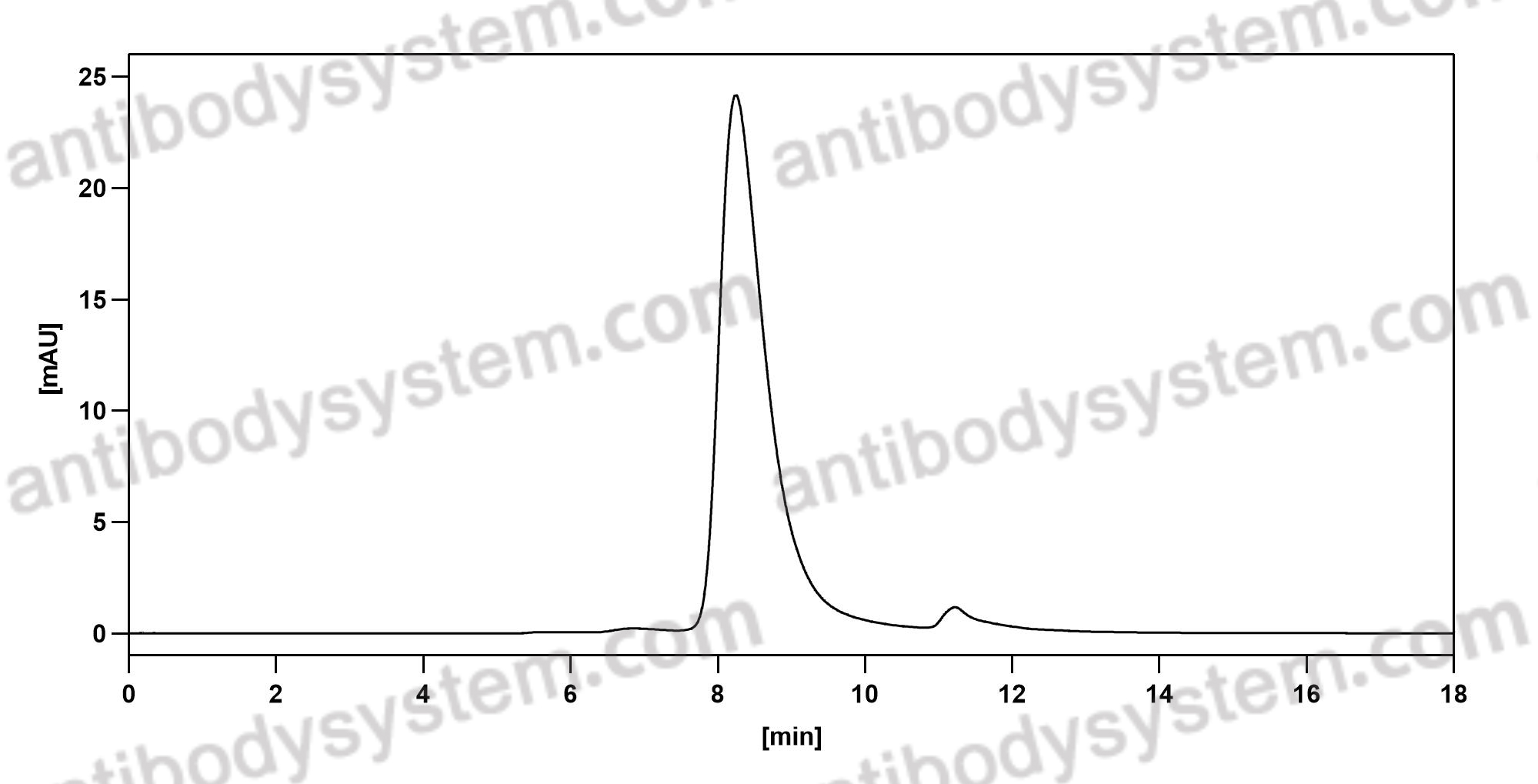
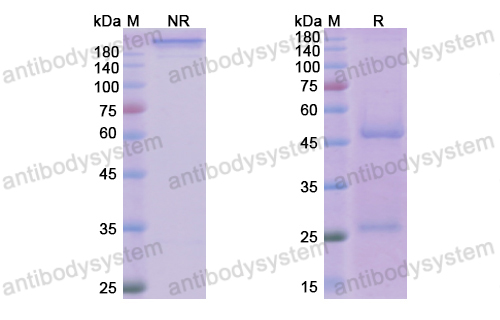
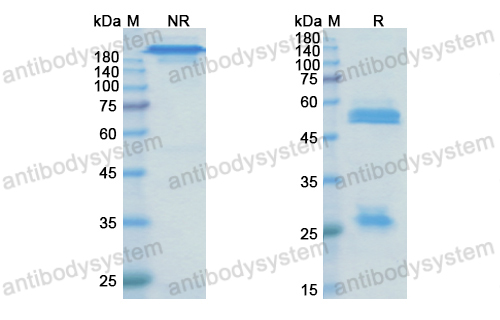
Guselkumab for the treatment of palmoplantar pustulosis, PMID: 32321322
Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial, PMID: 32178766
Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial, PMID: 32178765
Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial, PMID: 28057360
Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial, PMID: 28057361
A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial, PMID: 31887225
Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial, PMID: 31402114
A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial, PMID: 32880909
Guselkumab for the treatment of psoriasis, PMID: 29482382
Guselkumab for the Treatment of Psoriasis, PMID: 29470778
Guselkumab Efficacy after Withdrawal Is Associated with Suppression of Serum IL-23-Regulated IL-17 and IL-22 in Psoriasis: VOYAGE 2 Study, PMID: 31207232
Efficacy and Safety of Guselkumab, an Interleukin-23p19-Specific Monoclonal Antibody, Through One Year in Biologic-Naive Patients With Psoriatic Arthritis, PMID: 33043600
Guselkumab for plaque psoriasis, PMID: 31363311
Guselkumab: A Review in Moderate to Severe Plaque Psoriasis, PMID: 30467781
Guselkumab, PMID: 31643594
Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review, PMID: 32427307
[Guselkumab], PMID: 31200928
Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials, PMID: 31583255
Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial, PMID: 28635018
Guselkumab: the First Selective IL-23 Inhibitor for Active Psoriatic Arthritis in Adults, PMID: 33251833
Efficacy and Safety of Guselkumab in Japanese Patients With Palmoplantar Pustulosis: A Phase 3 Randomized Clinical Trial, PMID: 31268476
Guselkumab in the treatment of moderate-to-severe plaque psoriasis, PMID: 32314622
Impact of guselkumab, an interleukin-23 p19 subunit inhibitor, on enthesitis and dactylitis in patients with moderate to severe psoriatic arthritis: results from a randomised, placebo-controlled, phase II study, PMID: 32665433
Guselkumab, PMID: 29999977
Efficacy and Safety of Guselkumab, an Anti-interleukin 23 Monoclonal Antibody, for Palmoplantar Pustulosis: A Randomized Clinical Trial, PMID: 29417135
Continuous treatment with guselkumab maintains clinical responses through 4 years in patients with moderate-to-severe psoriasis: results from VOYAGE 1, PMID: 32660282
Guselkumab for the treatment of moderate-to-severe plaque psoriasis, PMID: 29478344
A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis, PMID: 29532693
Efficacy of guselkumab in a subpopulation with pustulotic arthro-osteitis through week 52: an exploratory analysis of a phase 3, randomized, double-blind, placebo-controlled study in Japanese patients with palmoplantar pustulosis, PMID: 32173916
Efficacy of Guselkumab Compared With Adalimumab and Placebo for Psoriasis in Specific Body Regions: A Secondary Analysis of 2 Randomized Clinical Trials, PMID: 29799960
Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study, PMID: 29893222
Guselkumab for the treatment of psoriasis - evidence to date, PMID: 31391856
Long-Term Efficacy of Guselkumab for the Treatment of Moderate-to-Severe Psoriasis: Results from the Phase 3 VOYAGE 1 Trial Through Two Years, PMID: 30124721
Guselkumab in the treatment of concomitant hidradenitis suppurativa, psoriasis, and Crohn's disease, PMID: 31389737
Tremfya™ (Guselkumab), PMID: 30888946
Improvement in Patient-Reported Outcomes (Dermatology Life Quality Index and the Psoriasis Symptoms and Signs Diary) with Guselkumab in Moderate-to-Severe Plaque Psoriasis: Results from the Phase III VOYAGE 1 and VOYAGE 2 Studies, PMID: 30417277
A safety evaluation of guselkumab for the treatment of psoriasis, PMID: 29897790
Evaluating guselkumab: an anti-IL-23 antibody for the treatment of plaque psoriasis, PMID: 31354244
Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: Results from the VOYAGE 1 and VOYAGE 2 trials, PMID: 31809827
Guselkumab: First Global Approval, PMID: 28819723
Guselkumab for the Treatment of Psoriasis: A Review of Phase III Trials, PMID: 28639011
Maintenance of Response Through up to 4 Years of Continuous Guselkumab Treatment of Psoriasis in the VOYAGE 2 Phase 3 Study, PMID: 32910434
Guselkumab: human monoclonal antibody against interleukin-23 for moderate to severe psoriasis, PMID: 29771254
Efficacy of in-class interleukin-23 inhibitor switching: risankizumab following guselkumab failure in moderate-to-severe psoriasis treatment, PMID: 32998185
Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics, PMID: 30772098
Guselkumab is superior to fumaric acid esters in patients with moderate-to-severe plaque psoriasis who are naive to systemic treatment: results from a randomized, active-comparator-controlled phase IIIb trial (POLARIS), PMID: 31705526
Clinical Utility of Guselkumab in the Treatment of Moderate-to-Severe Plaque Psoriasis, PMID: 33488109
Psoriasis Drug Guselkumab Improved Psoriatic Arthritis Symptoms, PMID: 32453367
IL-23 inhibitor guselkumab shows promise for PsA, PMID: 32265523
Guselkumab: Short-term effectiveness and safety in real clinical practice, PMID: 32227659
Biologics targeting IL-23 in moderate-to-severe psoriasis: lessons learned from real-world use., PMID:40522209
Psoriasis vulgaris in patients with a recent history of neoplasia: safety of interleukin-23 inhibitors. A multicentre retrospective study., PMID:40521671
Safety of guselkumab and ustekinumab treatment in patients with moderate-to-severe plaque psoriasis combined with latent tuberculosis or inactive hepatitis B virus infection: A retrospective multicenter observational study., PMID:40482822
Understanding Psoriasis Patient Preferences for Biologic Dosing Frequencies: Insights From a Patient Survey., PMID:40476147
Positioning Guselkumab in The Treatment Algorithm of Patients with Crohn's Disease., PMID:40470513
Comparative Efficacy of Different Targeted Therapies in Patients With Moderate-to-Severe Ulcerative Colitis: Systematic Review/Network Meta-Analysis and Mechanistic Overview., PMID:40468857
Guselkumab Is Effective for the Management of Scalp Psoriasis in Both Biologic-Naïve and Biologic-Experienced Patients: Results From a Real-World, Retrospective Study., PMID:40468568
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Commentary: Guselkumab binding to CD64+ IL-23-producing myeloid cells enhances potency for neutralizing IL-23 signaling., PMID:40463376
Guselkumab (Tremfya) - an IL-23 antagonist for Crohn's disease., PMID:40459404
The effect of IL-17 and IL-23 ınhibitors on hematological ınflammatory parameters in patients with psoriasis vulgaris., PMID:40455345
A Matching-Adjusted Indirect Comparison of Guselkumab and Secukinumab in Patients with Psoriatic Arthritis Over 52 Weeks., PMID:40450642
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Comparative Efficacy of Ustekinumab and Guselkumab in Improving Itch in Severe Psoriasis Patients., PMID:40432363
Emerging Therapies for Palmoplantar Pustulosis with a Focus on IL-23 Inhibitors., PMID:40429269
Network Meta-Analysis: Efficacy of Biological Therapies and Small Molecules as Maintenance Therapy in Ulcerative Colitis., PMID:40407729
Real-World Utilization of Biologic and Targeted Synthetic Disease-Modifying Anti-rheumatic Drugs in Psoriatic Arthritis and Axial Spondyloarthritis: Insights from Sweden and Germany., PMID:40402376
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Early-Stage Limited Cutaneous Systemic Sclerosis Successfully Treated With Guselkumab: Possibility of Early Intervention in the Pathogenesis., PMID:40401802
Interleukin-23p19 inhibitors for the treatment of moderate-to-severe psoriasis: an expert opinion of real-world evidence studies in Europe., PMID:40396610
With guselkumab, does the dual mechanisms to inhibit IL-23, help in ulcerative colitis?, PMID:40394835
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Guselkumab in Psoriatic Arthritis: Therapeutic Impact on Axial and Peripheral Involvement-Monocentric Real-World Evidence., PMID:40364181
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:40357993
Biologics for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-analysis., PMID:40329054
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis., PMID:40327280
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Novel Small-Molecule Treatment and Emerging Biological Therapy for Psoriasis., PMID:40299379
EBV-positive Hodgkin lymphoma with immunosuppressive microenvironment during IL-23 inhibitor therapy for psoriasis., PMID:40297411
Effectiveness and Safety of Guselkumab in Patients With Moderate-to-Severe Plaque Psoriasis in Real-World Practice in Korea: A Prospective, Multicenter, Observational, Postmarketing Surveillance Study., PMID:40293170
Efficacy and Safety of IL-17 and IL-23 Inhibitors in Elderly Patients With Plaque Psoriasis: A Real-World Study., PMID:40285439
New Interleukin-23 Antagonists' Use in Crohn's Disease., PMID:40283885
The role of interleukin inhibitors in the treatment of hidradenitis suppurativa; a systematic review of clinical trials., PMID:40268126
Targeting nail psoriasis: IL-17A inhibitors demonstrate site-specific superiority over IL-23 inhibitor in a 24-week dermoscopy-guided real-world cohort., PMID:40264783
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
Guselkumab: a safe treatment option for patients with comorbid psoriasis and multiple sclerosis., PMID:40248968
A Long-Term Real-Life Safety Study of Guselkumab in Psoriasis Patients with Infectious Comorbidities, Malignancies or Heart Disease: The EARLY Study., PMID:40238960
Predictive Factors for Super Responder Status and Long-Term Effectiveness of Guselkumab in Psoriasis: A Multicenter Retrospective Study., PMID:40238056
Effects of Guselkumab on the FIB-4 Index in Psoriasis Patients (EGIPT): A Three-Year Study., PMID:40237394
Unveiling the effectiveness and safety spectrum of biologic therapies in psoriasis: a three-year real-world analysis., PMID:40227059
Roflumilast Cream 0.3% for a Patient With Genital Psoriasis Refractory to Other Topical and Systemic Treatments: A Case Report., PMID:40212438
Treatment of Plaque Psoriasis with Guselkumab Reduces Systemic Inflammatory Burden as Measured by Neutrophil/Lymphocyte Ratio, Platelet/Lymphocyte Ratio, and Monocyte/Lymphocyte Ratio: A post hoc Analysis of Three Randomised Clinical Trials., PMID:40209685
Exploring the Impact of Guselkumab and Risankizumab on Psoriasis in HIV-Positive Patients: Insights From Four Italian Centers., PMID:40176606
Successful Guselkumab Treatment in a Patient with Comorbid Psoriasis and Amyotrophic Lateral Sclerosis: A Case Study and Literature Review., PMID:40166719
A Practical Guide to the Use of Guselkumab for Moderate-Severe Ulcerative Colitis., PMID:40162667
Comparative efficacy of pharmacologic interventions in ulcerative colitis: a network meta analysis., PMID:40156676
Real-world safety and effectiveness of guselkumab in patients with psoriasis: A post-marketing surveillance study through up to week 52 in Japan., PMID:40156259