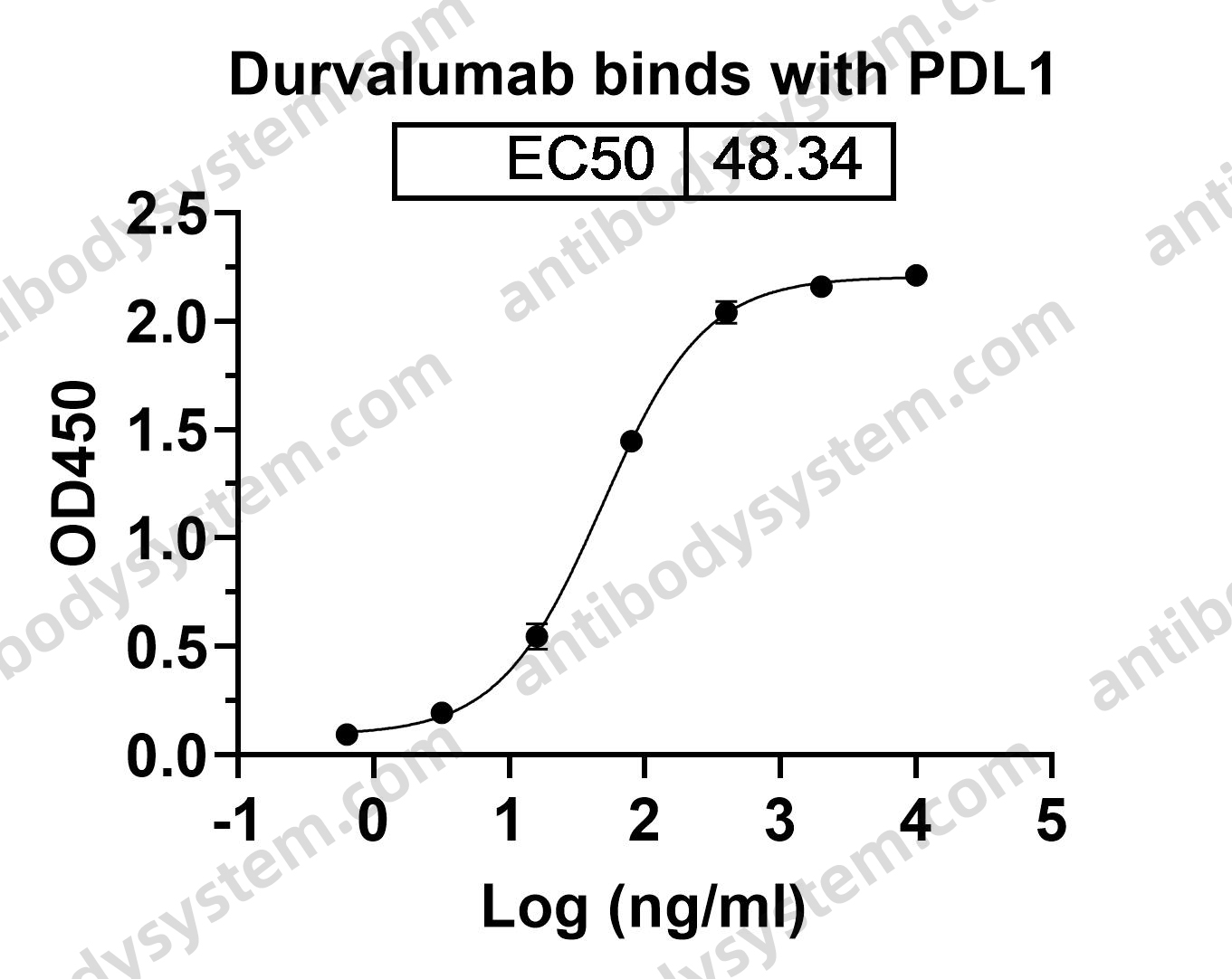
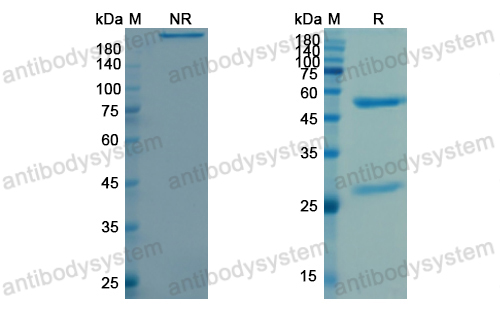
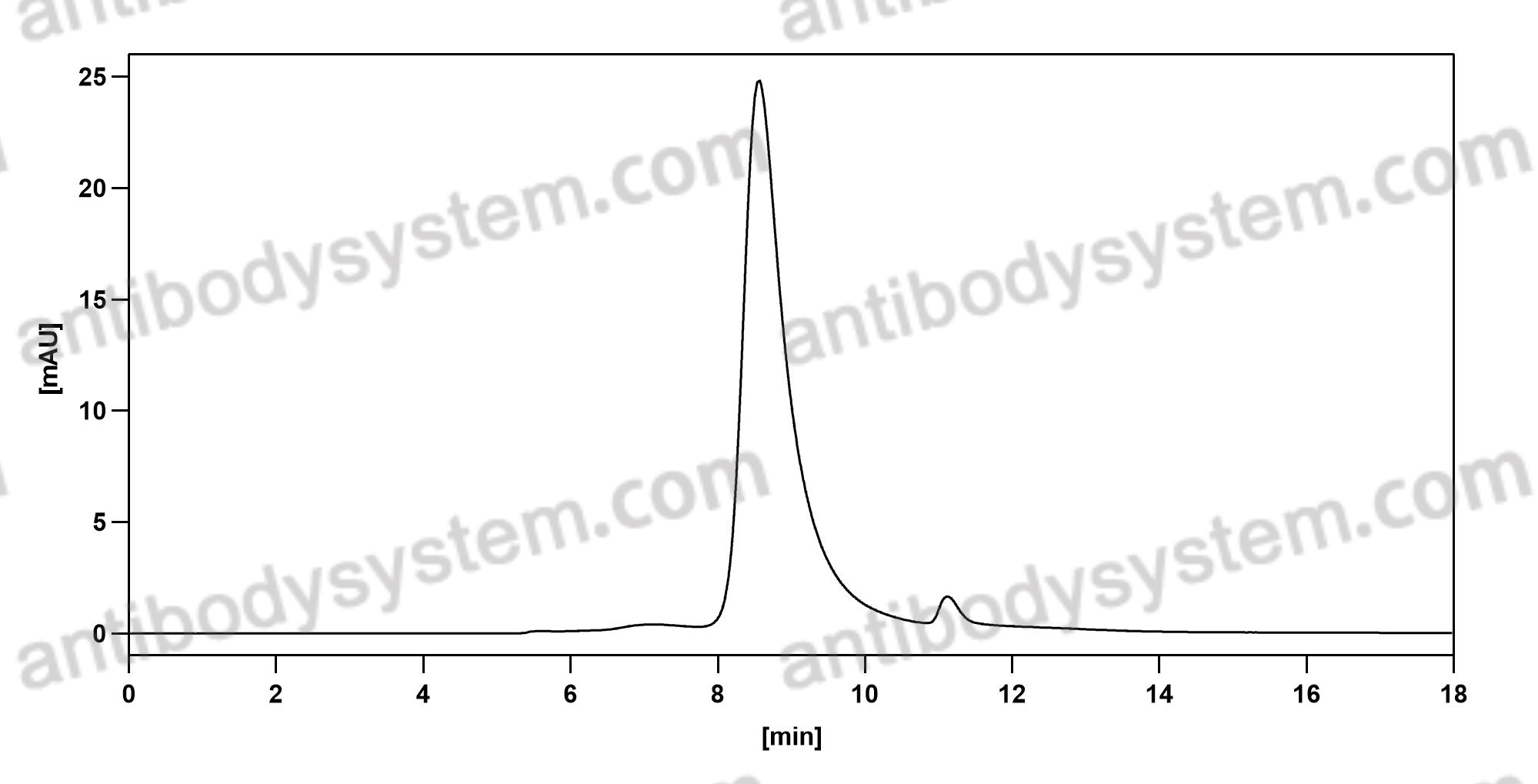
Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial, PMID: 31590988
Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer, PMID: 28885881
Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC, PMID: 30280658
Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC, PMID: 31622733
Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial, PMID: 32271377
Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial, PMID: 33285097
Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial, PMID: 33476803
Durvalumab for the treatment of non-small cell lung cancer, PMID: 31782989
Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study, PMID: 32771088
Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study, PMID: 32294530
A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study, PMID: 31095287
Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial, PMID: 32971005
Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial, PMID: 32209338
TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer, PMID: 32139298
Durvalumab, PMID: 31643480
Durvalumab in urothelial cancers, PMID: 29486607
Precision medicine phase II study evaluating the efficacy of a double immunotherapy by durvalumab and tremelimumab combined with olaparib in patients with solid cancers and carriers of homologous recombination repair genes mutation in response or stable after olaparib treatment, PMID: 32778095
ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer, PMID: 32201234
Durvalumab in cancer medicine: a comprehensive review, PMID: 31272242
Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial, PMID: 30383184
Durvalumab therapy following chemoradiation compared with a historical cohort treated with chemoradiation alone in patients with stage III non-small cell lung cancer: A real-world multicentre study, PMID: 33242835
Durvalumab, PMID: 29999970
Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study, PMID: 28817753
Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer, PMID: 32505077
Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study, PMID: 31601496
Safety evaluation of durvalumab for the treatment of non-small-cell lung cancer, PMID: 32357806
Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study, PMID: 29545095
Durvalumab for the treatment of non-small cell lung cancer, PMID: 29958099
Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial, PMID: 33462450
Durvalumab in Combination with Olaparib in Patients with Relapsed SCLC: Results from a Phase II Study, PMID: 31063862
Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single-arm, phase 2 trial with a safety run-in, PMID: 32888453
Durvalumab for urothelial carcinoma, non-small cell lung cancer, PMID: 31363310
Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial, PMID: 31318392
Osimertinib Plus Durvalumab versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC following Previous EGFR TKI Therapy: CAURAL Brief Report, PMID: 30763730
Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab, PMID: 28717238
Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma, PMID: 31676670
Population Pharmacokinetics of Durvalumab in Cancer Patients and Association With Longitudinal Biomarkers of Disease Status, PMID: 29243223
Patient-reported outcomes with first-line durvalumab plus platinum-etoposide versus platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase III study, PMID: 32961445
Impact of prior chemoradiotherapy-related variables on outcomes with durvalumab in unresectable Stage III NSCLC (PACIFIC), PMID: 33285469
Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations, PMID: 30514390
Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study, PMID: 26858122
Design and Rationale for a Phase III, Randomized, Placebo-controlled Trial of Durvalumab With or Without Tremelimumab After Concurrent Chemoradiotherapy for Patients With Limited-stage Small-cell Lung Cancer: The ADRIATIC Study, PMID: 31948903
Durvalumab for the Treatment of Locally Advanced, Unresectable, Stage III Non-Small Cell Lung Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal, PMID: 31814080
Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ), PMID: 32827847
Clinical Activity, Tolerability, and Long-Term Follow-Up of Durvalumab in Patients With Advanced NSCLC, PMID: 31228626
Durvalumab: a newly approved checkpoint inhibitor for the treatment of urothelial carcinoma, PMID: 30270097
Durvalumab: an investigational anti-PD-L1 monoclonal antibody for the treatment of urothelial carcinoma, PMID: 29416316
A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1-3 inhibitor, cediranib, in recurrent women's cancers with biomarker analyses, PMID: 31345267
Durvalumab for the management of urothelial carcinoma: a short review on the emerging data and therapeutic potential, PMID: 31040693
Durvalumab: a potential maintenance therapy in surgery-ineligible non-small-cell lung cancer, PMID: 29760563
Site-Selective Anti-PD-L1 Antibody-MMAE Conjugate for Enhanced NSCLC Therapy., PMID:40529092
Adding durvalumab to perioperative FLOT improves gastric cancer outcomes., PMID:40528045
A Prognostic Index for Advanced Biliary Tract Cancer Treated With Cisplatin, Gemcitabine and Durvalumab: The MAGIC-D Index., PMID:40525496
Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Patients With HCC Treated With Durvalumab Plus Tremelimumab., PMID:40515751
First-in-human phase I/II, open-label study of mRNA-2416 alone or combined with durvalumab in patients with advanced solid tumors and ovarian cancer., PMID:40515479
A Critical Review of Immunomodulation in the Management of Inoperable Stage III NSCLC., PMID:40507315
Erythrocytosis as an indicator of disease progression of small cell lung cancer: A case report., PMID:40502212
Ultrasensitive detection and tracking of circulating tumor DNA to predict relapse and survival in patients with locally advanced cervical cancer: phase III CALLA trial analyses., PMID:40500687
Conversion surgery following severe cytokine release syndrome induced by immune checkpoint inhibitors doublet in advanced hepatocellular carcinoma., PMID:40500480
Association of KRAS variants with survival and therapeutic outcomes in biliary tract cancers., PMID:40499463
Central Airway Invasion of Lung Squamous Cell Carcinoma Causing Bronchomediastinal Fistula and Mediastinitis., PMID:40491839
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
Cost-effectiveness of chemotherapy in advanced and recurrent endometrial cancer., PMID:40487854
Six-year survival after oral temozolomide maintenance therapy in limited-stage small cell lung cancer: A case report., PMID:40487755
Editorial Comment on "Safety of Partial and Radical Nephrectomy for Complex Locally Advanced Renal Cell Carcinoma After Neo-Adjuvant Immune Checkpoint Inhibition - Analysis from a Phase 1b Trial (Durvalumab +/- Tremelimumab)"., PMID:40484289
Durvalumab after concurrent chemoradiotherapy for sensitizing epidermal growth factor receptor-mutant stage III non-small cell lung cancer: A Japanese Real-World data analysis., PMID:40480013
Clinical features, treatment, and outcomes of anti-PD-L1 induced psoriasis., PMID:40474015
Association of candidate surrogate endpoints with overall survival in advanced biliary tract cancer., PMID:40473034
Immune-mediated enterocolitis is associated with immune checkpoint inhibitors: A pharmacovigilance study from the FDA Adverse Event Reporting System (FAERS) database., PMID:40465755
Perioperative Durvalumab in Gastric and Gastroesophageal Junction Cancer., PMID:40454643
A Multimodality Treatment Strategy for an Intrahepatic Cholangiocarcinoma and Complex Liver Resection with Vascular Reconstruction-A Video Vignette., PMID:40450171
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial., PMID:40450142
Differential safety profiles of durvalumab monotherapy and durvalumab in combination with tremelimumab in adult patients with advanced cancers., PMID:40447320
Author response to comment on "Phase 2 study of neoadjuvant durvalumab plus docetaxel, oxaliplatin, and S-1 with surgery and adjuvant durvalumab plus S-1 for resectable locally advanced gastric cancer"., PMID:40447315
Treatment of small cell lung cancer; advances and future prospects., PMID:40446464
Safety of Partial and Radical Nephrectomy for Complex Locally Advanced Renal Cell Carcinoma After Neo-Adjuvant Immune Checkpoint Inhibition: Analysis From a Phase 1b Trial (Durvalumab +/- Tremelimumab)., PMID:40441307
Durvalumab after sequential chemoradiotherapy in unresectable stage III non-small-cell lung cancer-final analysis from the phase II PACIFIC-6 trial., PMID:40435873
A novel approach to evaluate the therapeutic efficacy of durvalumab and tremelimumab combination therapy in hepatocellular carcinoma., PMID:40433908
Diagnostic and Therapeutic Challenges in an Older Patient With Concurrent Small-Cell Lung Carcinoma and Primary Duodenal Adenocarcinoma: A Case Report., PMID:40421353
Outcomes in Patients With Resectable Stage III NSCLC Who Did Not Have Definitive Surgery After Neoadjuvant Treatment-A Retrospective Analysis of the SAKK Trials 16/96, 16/00, 16/01, 16/08, and 16/14: A Brief Report., PMID:40420867
[Complete Response Achieved by Gemcitabine+Cisplatin+Durvalumab Therapy for Lymph Node Recurrence of Intraductal Cholangiocarcinoma-A Case Report]., PMID:40420374
Looking Toward the Future: Emerging Therapies for Hepatocellular Carcinoma., PMID:40416920
The Importance of Timing in Immunotherapy: A Systematic Review., PMID:40416194
Adjuvant Immunotherapy in Microsatellite Instability-High Colon Cancer: A Literature Review on Efficacy, Challenges, and Future Directions., PMID:40415356
Multicenter phase Ib/II study of second-line durvalumab and tremelimumab in combination with paclitaxel in patients with biomarker-selected metastatic gastric cancer., PMID:40399487
Laparoscopic Extended Segmentectomy 8 with Right Hepatic Vein Resection After Conversion Therapy for Advanced Intrahepatic Cholangiocarcinoma., PMID:40397343
Real-world survival outcomes, treatment patterns, and impact of PD-L1 expression among patients with unresectable, stage III NSCLC treated with CRT → durvalumab in Canada: The RELEVANCE study., PMID:40393235
Factors associated with reaching maintenance therapy in patients with advanced biliary tract cancer treated with durvalumab: Real-world results from a multicenter and multinational study., PMID:40387725
Factors predicting early recurrence in patients with unresectable stage III non-small cell lung cancer on durvalumab consolidation after chemoradiotherapy., PMID:40386716
A platform for SpyCatcher conjugation to native antibodies., PMID:40386161
Immune-mediated adverse events and overall survival with tremelimumab plus durvalumab and durvalumab monotherapy in unresectable HCC: HIMALAYA phase III randomized clinical trial., PMID:40384092
Durvalumab plus chemotherapy in advanced biliary tract cancer: 3-year overall survival update from the phase III TOPAZ-1 study., PMID:40381735
First-line durvalumab therapy alone or in combination with tremelimumab for metastatic head and neck squamous cell carcinoma: A cost-effectiveness analysis., PMID:40378108
Common Medical Comorbidities Influence Pneumonitis Risk After Chemoradiotherapy and Durvalumab Maintenance in Stage III Non-small Cell Lung Cancer., PMID:40374425
Implementing adjuvant immunotherapy following radical chemoradiotherapy for stage III non-small-cell lung cancer in UK clinical practice-Are the PACIFIC trial outcomes achievable in the real world?, PMID:40371096
Association of Sinonasal Symptoms and Disease With Immune Checkpoint Inhibitor Therapy., PMID:40370338
Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR)., PMID:40361370
A pharmacovigilance analysis of post-marketing safety of durvalumab., PMID:40360595
Equalizing prognostic disparities in KRAS-mutated stage III NSCLC patients: addition of durvalumab to combined chemoradiotherapy improves survival., PMID:40349418
Impact of durvalumab re-administration after moderate symptomatic pneumonitis in locally advanced non-small cell lung cancer., PMID:40349417