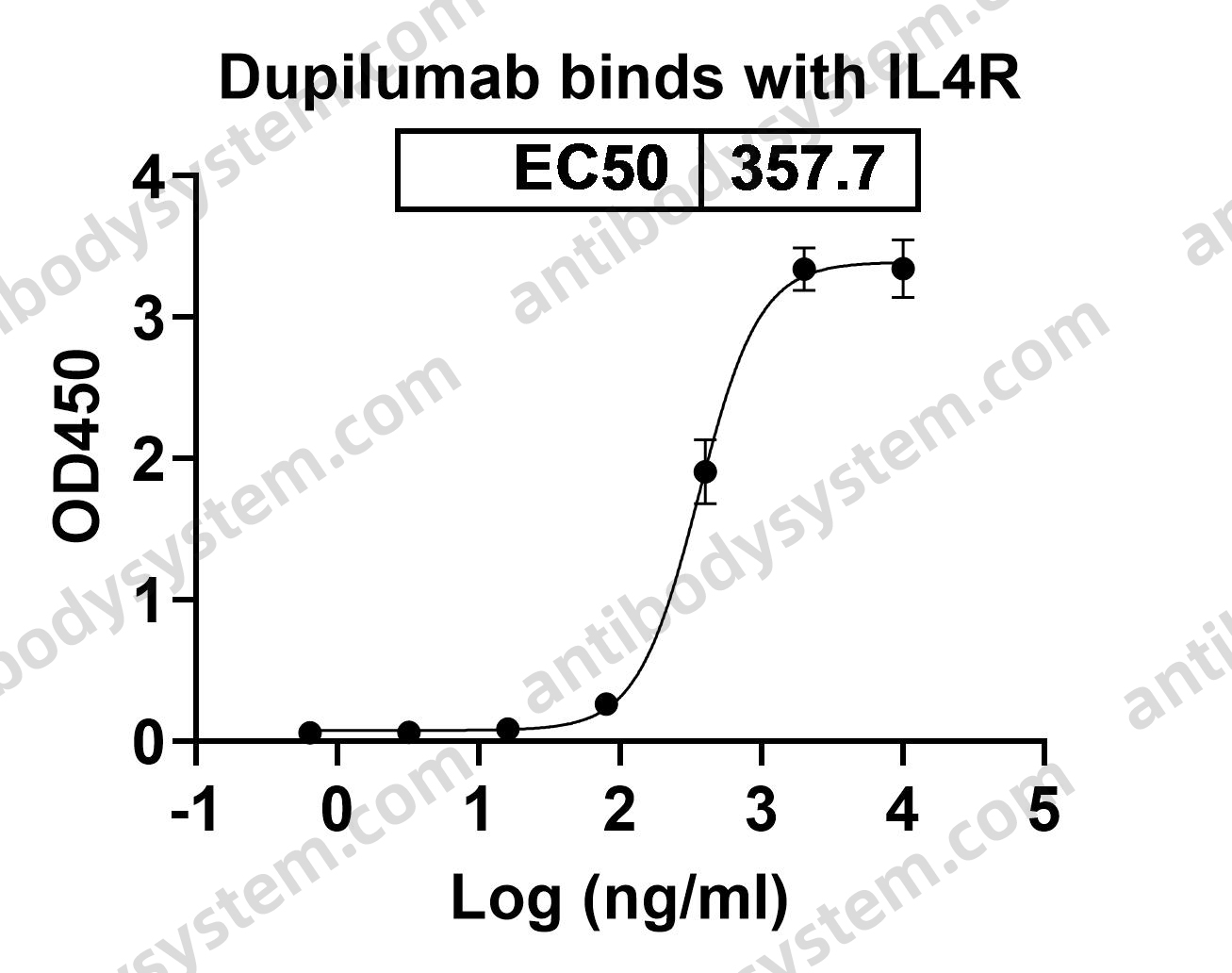
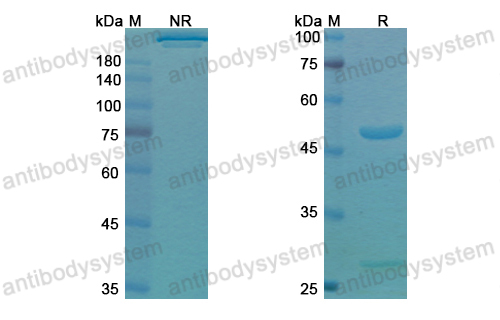
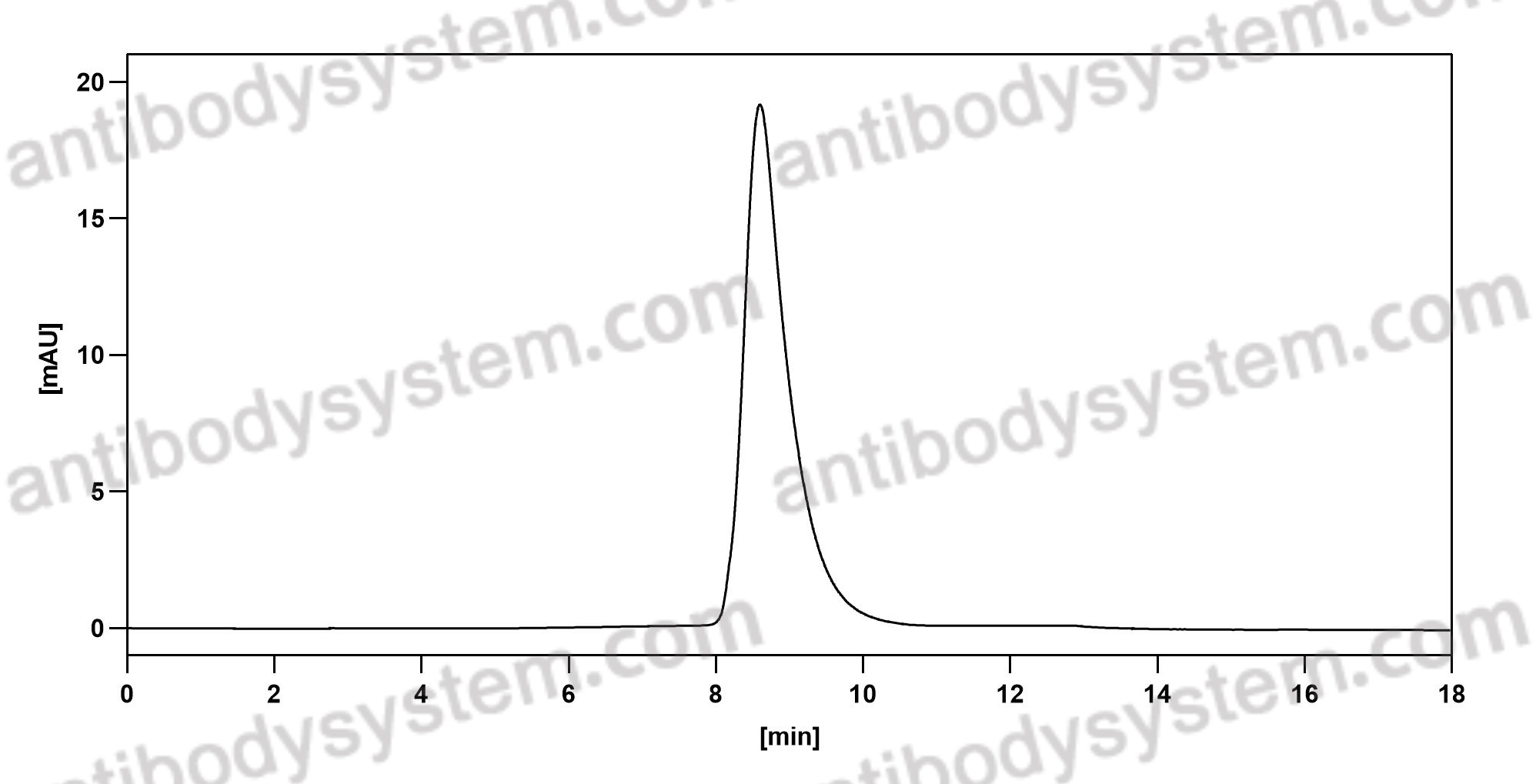
Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma, PMID: 29782217
Dupilumab for treatment of atopic dermatitis, PMID: 29557246
Mechanisms of Dupilumab, PMID: 31505066
Dupilumab: A review of its use in the treatment of atopic dermatitis, PMID: 29471919
Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials, PMID: 31543428
Dupilumab for the treatment of asthma, PMID: 28085503
Dupilumab treatment in adults with moderate-to-severe atopic dermatitis, PMID: 25006719
Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial, PMID: 28478972
Management of dupilumab-associated conjunctivitis in atopic dermatitis, PMID: 30873757
Efficacy and Safety of Dupilumab in Adolescents With Uncontrolled Moderate to Severe Atopic Dermatitis: A Phase 3 Randomized Clinical Trial, PMID: 31693077
Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma, PMID: 29782224
Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial, PMID: 32574587
Dupilumab for atopic dermatitis: evidence to date, PMID: 31210470
Dupilumab: Basic aspects and applications to allergic diseases, PMID: 32007360
Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis, PMID: 30194992
Dupilumab: A New Paradigm for the Treatment of Allergic Diseases, PMID: 29939132
Conjunctivitis in dupilumab clinical trials, PMID: 30851191
Dupilumab for prurigo nodularis: Case series and review of the literature, PMID: 31917498
Dupilumab and COVID-19: What should we expect?, PMID: 32362061
Dupilumab to Treat Type 2 Inflammatory Diseases in Children and Adolescents, PMID: 32157553
Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis, PMID: 32822798
Dupilumab: A Review in Chronic Rhinosinusitis with Nasal Polyps, PMID: 32240527
Dupilumab use in dermatologic conditions beyond atopic dermatitis - a systematic review, PMID: 31693426
Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis, PMID: 31593702
Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study, PMID: 31374300
Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma, PMID: 31521831
Dupilumab in the treatment of chronic rhinosinusitis with nasal polyposis, PMID: 32075470
Dupilumab in the treatment of asthma, PMID: 31218914
Dupilumab in persistent asthma with elevated eosinophil levels, PMID: 23688323
A Clinician's Guide to the Recognition and Management of Dupilumab-Associated Conjunctivitis, PMID: 31728936
Dupilumab (Dupixent) for Asthma, PMID: 32053330
Dupilumab treatment in moderate-to-severe atopic dermatitis: A systematic review and meta-analysis, PMID: 29472119
Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ), PMID: 29193016
Dupilumab for the treatment of adolescents with atopic dermatitis, PMID: 32720530
Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series, PMID: 32179082
Efficacy and Safety of Multiple Dupilumab Dose Regimens After Initial Successful Treatment in Patients With Atopic Dermatitis: A Randomized Clinical Trial, PMID: 31876900
Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis, PMID: 30488542
Real-world persistence with dupilumab among adults with atopic dermatitis, PMID: 32739313
Dupilumab: Clinical Efficacy of Blocking IL-4/IL-13 Signalling in Chronic Rhinosinusitis with Nasal Polyps, PMID: 32440101
IL-4Rα Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis, PMID: 31252032
Eye Complications During Dupilumab Treatment for Severe Atopic Dermatitis, PMID: 30653240
[Experience of using dupilumab in the treatment of severe asthma], PMID: 33346468
▼Dupilumab for atopic dermatitis, PMID: 29545264
[Key-studies on dupilumab], PMID: 30093077
Dupilumab Provides Favorable Safety and Sustained Efficacy for up to 3 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis, PMID: 32557382
Dupilumab for bullous pemphigoid with intractable pruritus, PMID: 32045153
A review of dupilumab in the treatment of atopic diseases, PMID: 30785362
Dupilumab for the Treatment of Recalcitrant Bullous Pemphigoid, PMID: 30140849
Treatment of Netherton Syndrome With Dupilumab, PMID: 31995125
Dupilumab, severe asthma airway responses, and SARS-CoV-2 serology, PMID: 32767400
Dupilumab Efficacy in Children With Eosinophilic Esophagitis With Prior Swallowed Topical Corticosteroid Use: A Subgroup Analysis., PMID:40531526
Psoriasis Risk in Patients With Atopic Dermatitis Treated With Dupilumab., PMID:40531497
Eosinophil and Cationic Protein Levels in Patients Treated With Dupilumab: A Real-Life Study., PMID:40530362
Leukocytoclastic Vasculitis With Eosinophilia in a Patient Receiving Dupilumab Therapy., PMID:40530217
Adverse Effects of Dupilumab for Chronic Rhinosinusitis With Nasal Polyposis: Real-World Single Institution Experience., PMID:40525458
Anti-IL-4Rα aptamer reduces IL-4 signalling and formation of nasal polyps., PMID:40524931
Four Cases of Prurigo Nodularis Requiring Nemolizumab due to Persistent Pruritus or Lesions after Dupilumab Treatment., PMID:40524402
Tape Strip Profiling of Checkpoint Inhibitor-Associated Dermatitis Highlights Pan-T-Cell Activation: A Pilot Study., PMID:40519867
[Analysis of the clinical efficacy and safety of dupilumab in the treatment of patients with moderate to severe atopic dermatitis complicated with asthma]., PMID:40518414
The Efficacy of Dupilumab in Pemphigus Vulgaris., PMID:40514796
Clinical response and remission in patients treated with dupilumab for severe asthma: Results from the nationwide Danish Severe Asthma Register., PMID:40513967
Recalcitrant Grover's disease treated with dupilumab: two long-term cases and review of the literature., PMID:40509692
Efficacy of Upadacitinib in Treating Alopecia Areata, Atopic Dermatitis, and Th1 Comorbidities in Pediatric Patients: A Comprehensive Case Series and Literature Review., PMID:40507644
Dupilumab-Induced Remission in Chronic Rhinosinusitis with Nasal Polyps and Comorbid Asthma: A 24-Month Study., PMID:40507416
Interleukin-4 and -13 Gene Expression Profiles in Immune-Related Bullous Pemphigoid Indicate Efficacy of IL-4/IL-13 Inhibitors., PMID:40507326
Dupilumab in an adolescent with eosinophilic esophagitis and eosinophilic duodenitis, a valuable treatment option., PMID:40504588
Dupilumab treatment has no effect on the nasal microbiome in patients with NSAID-exacerbated respiratory disease: a longitudinal pilot study., PMID:40503236
STAT6 gain-of-function disease: p.D519N is a new disease-causing variant that responds well to dupilumab treatment., PMID:40502541
Paradoxical Effects of Dupilumab in ABPA: Resolution of Mucus Plugging but Induction of Eosinophilic Pneumonia., PMID:40499647
Auditory and Ocular Manifestations in Pediatric Vitiligo., PMID:40498511
Dupilumab and Blood Eosinophilia: A Disease-Specific Phenomenon?, PMID:40492692
[Application and research progress of anti-IL-4α monoclonal antibody dupilumab in the treatment of type 2 asthma]., PMID:40491151
Eosinophilic esophagitis in children: new kids on the block., PMID:40490426
Aspirin-Exacerbated Respiratory Disease in the era of biologics., PMID:40490219
Drug-related visual blurring: findings from the U.S. Food and Drug Administration Adverse Event Reporting System database., PMID:40490170
Wells syndrome: emerging triggers and treatments- an updated systematic review., PMID:40488888
Effectiveness of Dupilumab Treatment in Patients With Type 2 Inflammation: A Real-Life Integrated Experience., PMID:40487737
Dupilumab-Induced Granuloma Annulare: A Challenging Adverse Effect of Atopic Dermatitis Treatment., PMID:40487493
Dupilumab Improves Histopathologic Features in Patients With Eosinophilic Esophagitis: LIBERTY EoE TREET Study Results., PMID:40487268
Advancements in Biologic Therapies for Pediatric Asthma: Emerging Therapies and Future Directions., PMID:40486460
Cost-utility analysis of sequential therapies for pediatric-onset eosinophilic esophagitis., PMID:40485534
A nationwide experience of biological treatments in children with eosinophilic esophagitis., PMID:40483369
Reply to "Considerations for enhancing dupilumab research in hypereosinophilic syndromes"., PMID:40483017
Considerations for enhancing dupilumab research in hypereosinophilic syndromes., PMID:40483016
Clinical and complete remission in patients with severe asthma with 24-month dupilumab treatment., PMID:40482373
Long-term effectiveness and safety of dupilumab in severe asthma: a 5-year real-world multicenter study., PMID:40480546
Real-World Efficacy and Safety of Dupilumab Retreatment in Adults With Relapsed Atopic Dermatitis: A Retrospective Study From China., PMID:40474541
Efficacy and glucocorticoid sparing of dupilumab in IgG4-related disease: a case series and literature review., PMID:40470558
Angiolymphoid hyperplasia with eosinophilia treated with dupilumab in a biologic-naïve patient., PMID:40466455
Effect of dupilumab for the therapy of atopic dermatitis and the effect on serum TARC, HBD2 and SEA-IgE levels and peripheral blood T helper cell subtypes., PMID:40462855
Real‑World Experience of 1 Year of Tralokinumab Treatment in Adult Patients with Atopic Dermatitis: A Single-Center Retrospective Study., PMID:40459712
Real-World Effectiveness and Safety of Dupilumab, Tralokinumab, and Upadacitinib in Patients with Atopic Dermatitis: A 52-Week International, Multicenter Retrospective Cohort Study., PMID:40459711
Dupilumab efficacy in patients with type 2 asthma and early Feno level reductions., PMID:40458676
Rapid Itch Improvement and Skin Clearance with Upadacitinib Versus Placebo (Measure Up 1 and Measure Up 2) and Versus Dupilumab (Heads Up): Results from Three Phase 3 Clinical Trials in Patients with Moderate-to-Severe Atopic Dermatitis., PMID:40457140
An unbiased tissue transcriptome analysis identifies potential markers for skin phenotypes and therapeutic responses in atopic dermatitis., PMID:40456762
[Not Available]., PMID:40456219
[Not Available]., PMID:40456218
Dupilumab Treatment Is Associated With Clinical Improvement and a Shift Toward a Health-Associated Nasal Passage Microbiota in Diffuse Type 2 Chronic Rhinosinusitis., PMID:40452334
Infectious complications of atopic dermatitis, an update., PMID:40447099
[Translated article] Exploring New Horizons in Allergic Contact Dermatitis Treatment: The Role of Emerging Therapies., PMID:40446917