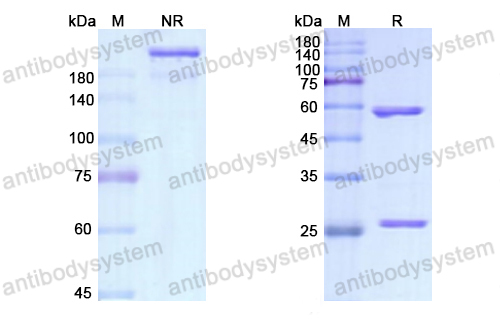
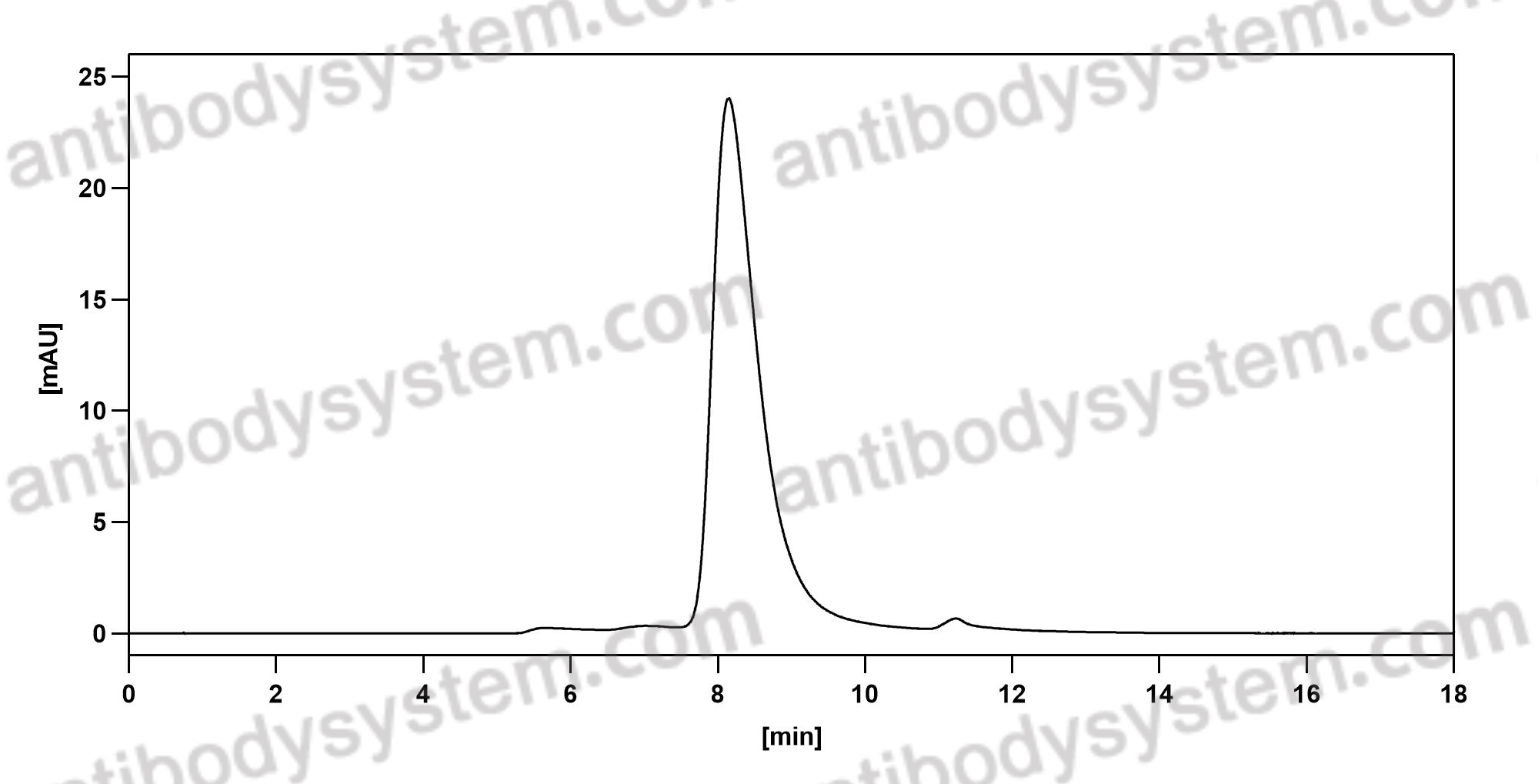
Brodalumab, PMID: 29261896
The safety of brodalumab for the treatment of psoriasis, PMID: 32053396
Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis, PMID: 26422722
Brodalumab: A Review of Safety, PMID: 29562088
Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials, PMID: 33106286
Brodalumab: A Review in Moderate to Severe Plaque Psoriasis, PMID: 29516365
Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials, PMID: 31583255
Brodalumab, PMID: 31643345
Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review, PMID: 32427307
Brodalumab for the treatment of plaque psoriasis: up-to-date, PMID: 30831036
[Brodalumab], PMID: 31204123
A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis, PMID: 29532693
Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial, PMID: 31175909
Brodalumab, PMID: 29999957
Brodalumab in the treatment of chronic plaque psoriasis, PMID: 32463723
Brodalumab in psoriasis: evidence to date and clinical potential, PMID: 31024633
Brodalumab: A new way to inhibit IL-17 in psoriasis, PMID: 32285564
Brodalumab for the treatment of psoriasis, PMID: 27718760
The Use of Brodalumab in Three Patients with Psoriasis and Psychiatric Comorbidities, PMID: 33488920
Recapture Rate of Brodalumab in Patients With a Lapse in Treatment, PMID: 32272515
Two-Year US Pharmacovigilance Report on Brodalumab, PMID: 33337520
Brodalumab for the Treatment of Moderate-to-Severe Plaque Psoriasis: An Evidence Review Group Evaluation of a NICE Single Technology Appraisal, PMID: 30112635
One-Year Pharmacovigilance Update of Brodalumab, PMID: 32845586
Malignancy Rates in Brodalumab Clinical Studies for Psoriasis, PMID: 32207067
Brodalumab for the Treatment of Psoriasis: A Review of Phase III Trials, PMID: 27221323
A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis, PMID: 26914406
Brodalumab to the Rescue: Efficacy and Safety of Brodalumab in Patients with Psoriasis and Prior Exposure or Inadequate Response to Biologics, PMID: 32533554
Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics, PMID: 30772098
Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review, PMID: 31939925
Brodalumab: the first anti-IL-17 receptor agent for psoriasis, PMID: 28650001
Brodalumab in the treatment of recalcitrant acrodermatitis continua of Hallopeau, PMID: 32920959
The comparative efficacy of brodalumab in patients with moderate-to-severe psoriasis: a systematic literature review and network meta-analysis, PMID: 29323542
Brodalumab: another helpful option for HIV-positive psoriatic patients?, PMID: 32584458
Brodalumab: short-term effectiveness and safety in real clinical practice, PMID: 32227476
Safety and Efficacy of Brodalumab for Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Meta-Analysis, PMID: 28197901
Paradoxical gastrointestinal effects of interleukin-17 blockers, PMID: 32719044
Efficacy of brodalumab in the treatment of scalp and nail psoriasis: results from three phase 3 trials, PMID: 32250714
Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents, PMID: 30017705
Spotlight on brodalumab in the treatment of moderate-to-severe plaque psoriasis: design, development, and potential place in therapy, PMID: 28744098
Pharmacokinetic drug evaluation of brodalumab for the treatment of psoriasis, PMID: 28460549
Brodalumab treatment in psoriasis patients with severe renal failure, PMID: 33228335
New Treatment Addressing the Pathogenesis of Psoriasis, PMID: 33050592
Long-term safety of brodalumab in Japanese patients with plaque psoriasis: An open-label extension study, PMID: 32275086
Brodalumab -an IL-17RA monoclonal antibody for psoriasis and psoriatic arthritis, PMID: 25985813
Brodalumab for the treatment of moderate-to-severe plaque-type psoriasis: a real-life, retrospective 24-week experience, PMID: 32916767
Comparison of Biologics and Oral Treatments for Plaque Psoriasis: A Meta-analysis, PMID: 32022825
Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis, PMID: 30181299
Psoriasis pathogenesis and the development of novel targeted immune therapies, PMID: 28887948
Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1), PMID: 32286683
Brodalumab success in patients with moderate-to-severe psoriasis who failed previous interleukin-17A inhibitors, PMID: 33221464
Dose modulation strategies in psoriatic patients: real-world comparison between secukinumab and brodalumab for up to one year after dose spacing., PMID:40499920
Severity of psoriasis and its impact on patient-reported outcomes (PROs): real world evidence with brodalumab 210 mg from the LIBERO study., PMID:40485500
Safety and efficacy of IL-17 inhibitors in patients with comorbid multiple sclerosis/multiple Sclerosis-like syndrome: a systematic review., PMID:40459586
Severe paradoxical generalized pustular psoriasis induced by adalimumab biosimilar successfully treated with brodalumab., PMID:40457932
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Switching to brodalumab after failure of IL-17A inhibitors in psoriatic patients: A real-life multicentre study., PMID:40439441
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Analysis and mining of brodalumab adverse events based on FAERS database., PMID:40414978
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
Successful treatment of localized scleroderma using brodalumab: A case report., PMID:40375964
IL-17C and Pityriasis Rubra Pilaris: Successful Treatment of Erythroderma With Brodalumab., PMID:40344230
Emerging manifestations of IL-17 immunomodulation in the gastrointestinal tract., PMID:40319948
The role of interleukin inhibitors in the treatment of hidradenitis suppurativa; a systematic review of clinical trials., PMID:40268126
Genetic Polymorphisms in Psoriasis: Investigating Genetic Variations for Precise Profiling of Response to Brodalumab in Real-Life Clinical Practice., PMID:40246146
BRodalumab effectiveness in psoriatic patients that experienced anti-IL17 Intra-Class failure: a multicentre, retrospective study (BRIC - Study) IL-PSO (Italian Landscape Psoriasis)., PMID:40233156
Summary of Research: Comparable Efficacy and Safety of Brodalumab in Obese and Nonobese Patients with Psoriasis: Analysis of Two Randomized Controlled Trials., PMID:40156694
Identifying transcriptomic predictors of brodalumab response in psoriasis using CART analysis., PMID:40140063
Real-world experience with brodalumab in a Portuguese cohort of patients with moderate-to-severe psoriasis., PMID:40124773
Brodalumab for Darier Disease: A Case Series Highlighting IL-17 Inhibition as a Potential Treatment., PMID:40119603
Risk of Herpes Zoster and Postherpetic Neuralgia in Patients with Psoriasis Treated with Biologics: A nationwide study using target trial emulation framework., PMID:40112174
Brodalumab for the treatment of palmoplantar psoriasis., PMID:40105777
Brodalumab for Plaque Psoriasis in the Canadian Real-World Setting: A Retrospective Cohort Analysis of up to 4 Years., PMID:40100536
Assessing brodalumab in the treatment of primary sclerosing cholangitis (SABR-PSC pilot study): protocol for a single-arm, multicentre, pilot study., PMID:40032516
Safety of Interleukin Inhibitors in Psoriatic Patients with Latent Tuberculosis Infection Without Chemoprophylaxis: A Systematic Review., PMID:40026108
Efficacy and Safety of Interleukin-17 and Janus Kinase Inhibitors in Ankylosing Spondylitis: A Systematic Review and Network Meta-Analysis., PMID:40010328
A case of systemic sclerosis accompanying psoriasis successfully treated with brodalumab., PMID:40008486
Effectiveness of Brodalumab for the Treatment of Moderate-to-Severe Psoriasis: A Retrospective, Real-World Multicenter Study with a Focus on Obese and Multi-Failure Patients-IL PSO (Italian Landscape Psoriasis)., PMID:40004618
Indirect Comparison Between Bimekizumab and Brodalumab for the Management of Moderate to Severe Psoriasis: A 36-Week Real-Life Study., PMID:39982649
PSO-TARGET: a New Tool to Identify the Therapeutic Expectations of Psoriasis Patients Treated with Biologics., PMID:39979765
Intra-class switch among interleukin-17 inhibitors for the treatment of plaque psoriasis: a single-center experience., PMID:39969045
Effectiveness, speed of action and safety of brodalumab in elderly psoriasis patients: a multicenter real-world study - IL PSO (Italian Landscape Psoriasis)., PMID:39914798
Lichenoid drug eruption during treatment with brodalumab for psoriasis., PMID:39898382
Interleukin-17: A pleiotropic cytokine implicated in inflammatory, infectious, and malignant disorders., PMID:39875232
Real-world switching patterns and associated characteristics in patients with psoriasis treated with biologics., PMID:39873811
A call to action regarding the Risk Evaluation and Mitigation Strategy program for brodalumab., PMID:39863170
A real-world Pharmacovigilance study of brodalumab based on the FDA adverse event reporting system., PMID:39824966
INDIVIDUAL ARTICLE: Psoriasis and Obesity: Optimizing Pharmacologic Treatment and Lifestyle Interventions., PMID:39761151
Risk of Candida and overall fungal infections with interleukin-17 inhibitors in the treatment of hidradenitis suppurativa: A systematic review and meta-analysis., PMID:39710114
Efficacy and Safety of Tumor Necrosis Factor Inhibitors, Interleukin-17 Inhibitors, and Janus Kinase Inhibitors in Patients with Non-Radiographic Axial Spondyloarthritis: A Systematic Review and Network Meta-Analysis., PMID:39657601
Biologics Use for Psoriasis during Pregnancy and Its Related Adverse Outcomes in Pregnant Women and Newborns: Findings from WHO Pharmacovigilance Study., PMID:39626647
Brodalumab: Six-Year US Pharmacovigilance Report., PMID:39589679
Systematic review of biologic use for psoriasis in HIV-positive individuals from 2018 to 2024., PMID:39537858
Psoriasis in Childbearing Age: A Real-Life, Retrospective, Single-Center Study on Anti-IL17 and IL-23 Agents., PMID:39518540
Brodalumab Is Effective for Psoriasis Patients with Difficult-To-Treat Body Regions: Results from an Observational Clinical Study., PMID:39504944
Interleukin-17 inhibitors for the management of severe rosacea., PMID:39494973
Prescribing Pattern and Safety Profile of Biological Agents for Psoriasis in Real-World Practice: A Four-Year Calabrian Pharmacovigilance Analysis., PMID:39458658
The Safety and Efficacy of Interleukin-17 Inhibitors for Hidradenitis Suppurativa: A Systematic Review and Meta-Analysis., PMID:39445454
Short term efficacy of biological treatment for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis., PMID:39424649
The Effects of Brodalumab on the Fungal Microbiome in Patients with Psoriasis., PMID:39408568
Factors Affecting Treatment Persistence in Japanese Patients with Psoriasis Prescribed Biologics: A Real-World Study Using an Insurance Claim Database., PMID:39407051