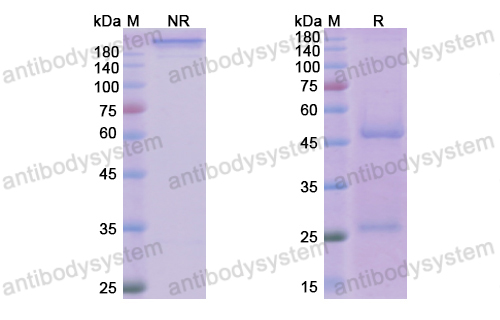
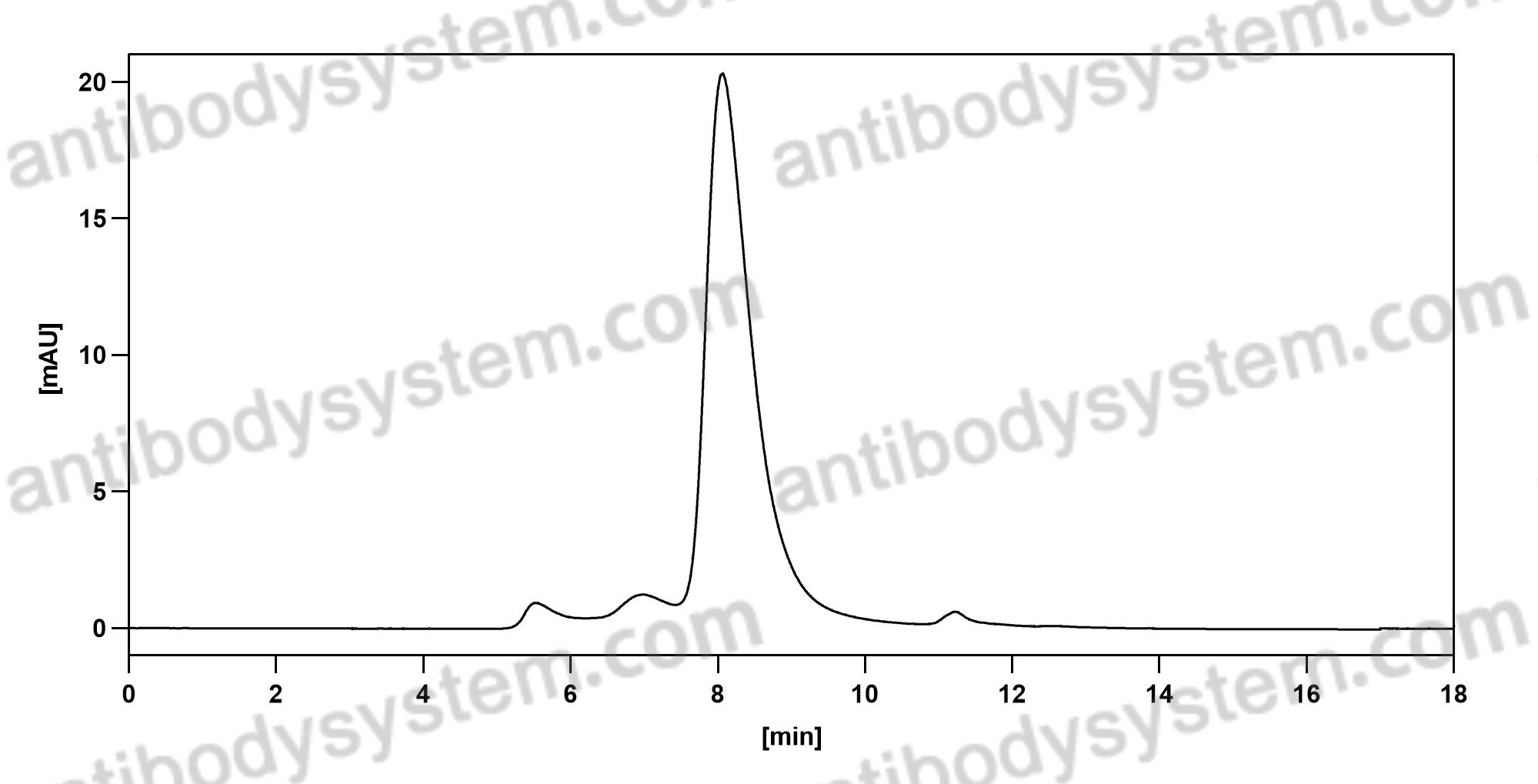
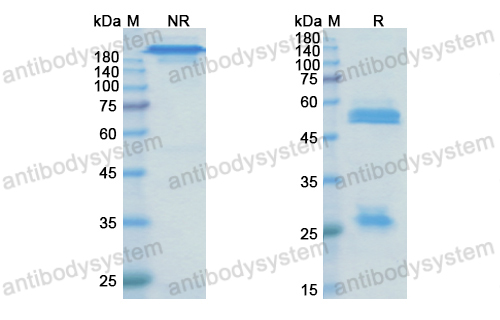
Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma, PMID: 32034960
Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials, PMID: 25736990
Reslizumab, PMID: 31643704
Reslizumab, PMID: 29999862
Reslizumab (Cinqair) [Internet], PMID: 29356467
Reslizumab in Eosinophilic Asthma: A Review, PMID: 28421429
Mabs for treating asthma: omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, PMID: 32401603
Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid-dependent asthma: results from two phase 3, randomised, double-blind, placebo-controlled trials, PMID: 32066536
Reslizumab in the treatment of severe eosinophilic asthma: an update, PMID: 29554826
Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts, PMID: 27018175
Update on reslizumab for eosinophilic asthma, PMID: 26372797
Reslizumab: First Global Approval, PMID: 27125787
Reslizumab for Treating Asthma with Elevated Blood Eosinophils Inadequately Controlled by Inhaled Corticosteroids: An Evidence Review Group Perspective of a NICE Single Technology Appraisal, PMID: 29582406
The effectiveness of Reslizumab in severe asthma treatment: a real-world experience, PMID: 31861993
Efficacy of Reslizumab Treatment in Exacerbation-Prone Patients with Severe Eosinophilic Asthma, PMID: 32562877
Reslizumab in the treatment of inadequately controlled asthma in adults and adolescents with elevated blood eosinophils: clinical trial evidence and future prospects, PMID: 28683596
Reslizumab in the management of poorly controlled asthma: the data so far, PMID: 27621657
Efficacy of Intravenous Reslizumab in Oral Corticosteroid-Dependent Asthma, PMID: 31626990
Reslizumab: Maintenance treatment for eosinophilic asthma inadequately controlled on corticosteroids, PMID: 27458609
Reslizumab as add-on therapy in patients with refractory asthma, PMID: 32273395
Pharmacokinetic/pharmacodynamic profile of reslizumab in asthma, PMID: 29268638
New treatments for chronic urticaria, PMID: 31446134
Impact of reslizumab on outcomes of severe asthmatic patients: current perspectives, PMID: 30147386
Anti-IL5 therapies for asthma, PMID: 28933516
Reslizumab and Eosinophilic Asthma: One Step Closer to Precision Medicine?, PMID: 28344579
Safety of Reslizumab in Uncontrolled Asthma with Eosinophilia: A Pooled Analysis from 6 Trials, PMID: 31404668
Safety of humanized monoclonal antibodies against IL-5 in asthma: focus on reslizumab, PMID: 29486600
Asthma in adults: Principles of treatment, PMID: 31690379
The efficacy and safety of reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: A systematic review and meta-analysis, PMID: 27435534
Reslizumab Compared with Benralizumab in Patients with Eosinophilic Asthma: A Systematic Literature Review and Network Meta-Analysis, PMID: 30217529
Efficacy of Reslizumab for Eosinophilic Esophagitis, PMID: 29985878
Update on new biologics for intractable eosinophilic asthma: impact of reslizumab, PMID: 29780238
Precision medicine in asthma: linking phenotypes to targeted treatments, PMID: 29036018
Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma - a systematic review and meta-analysis, PMID: 30533206
Diagnosis and Management of Asthma in Adults: A Review, PMID: 28719697
Effect of intravenously administered reslizumab on spirometric lung age in patients with moderate-to-severe eosinophilic asthma, PMID: 31262379
Reslizumab and mepolizumab for moderate-to-severe poorly controlled asthma: an indirect comparison meta-analysis, PMID: 31686556
Higher Binding Affinity and in vitro Potency of Reslizumab for Interleukin-5 Compared With Mepolizumab, PMID: 30661320
Clinical Outcomes and Health-Care Resource Use Associated With Reslizumab Treatment in Adults With Severe Eosinophilic Asthma in Real-World Practice, PMID: 33333058
Weight-adjusted Intravenous Reslizumab in Severe Asthma with Inadequate Response to Fixed-Dose Subcutaneous Mepolizumab, PMID: 28915080
Molecular Targets for Biological Therapies of Severe Asthma, PMID: 33329598
Dupilumab for the treatment of asthma, PMID: 28085503
Efficacy and safety of reslizumab in patients with moderate to severe eosinophilic asthma, PMID: 25578680
Update in Asthma and Airway Inflammation 2018, PMID: 31026407
Biological therapies for eosinophilic asthma, PMID: 29938543
A cost-effectiveness analysis of reslizumab in the treatment of poorly controlled eosinophilic asthma, PMID: 30003833
[Clinical and economic analysis of Reslizumab use in the treatment of patients with severe allergic eosinophilic asthma], PMID: 32598589
Targeted Molecular Therapies in Allergy and Rhinology, PMID: 33138725
Cost-utility analysis of reslizumab for patients with severe eosinophilic asthma inadequately controlled with high-dose inhaled corticosteroids and long-acting β 2-agonists in South Korea, PMID: 30964365
Population Pharmacokinetic and Pharmacokinetic/Pharmacodynamic Modeling of Weight-Based Intravenous Reslizumab Dosing, PMID: 32333684
Cost-effectiveness of mepolizumab vs anti-interleukin-5/5r biologic therapies for the treatment of adults with severe asthma with an eosinophilic phenotype: a Chilean healthcare system perspective., PMID:40528625
Steroid-sparing benefits of biologic use in hypereosinophilic syndrome and substantial disease burden across subtypes., PMID:40486501
Exploratory analysis of the economically justifiable price of reslizumab for asthma severe in Colombia., PMID:40481431
Multi-trait Analysis of GWAS Expands Eosinophilic Esophagitis Genetic Susceptibility and Polygenic Risk Scores., PMID:40470207
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Eosinophilic esophagitis: An allergy and immunology perspective on the updated guidelines., PMID:40339609
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
Efficacy of Biologics in Reducing Exacerbations Requiring Hospitalization or an Emergency Department Visit in Patients with Moderate or Severe, Uncontrolled Asthma., PMID:40261563
Severe Asthma and Active SARS-CoV-2 Infection: Insights into Biologics., PMID:40149651
Adverse effects of biologics used to treat asthma., PMID:40099886
Distinct roles for thymic stromal lymphopoietin (TSLP) and IL-33 in experimental eosinophilic esophagitis., PMID:40060399
Effectiveness of biological therapy in severe asthma: a retrospective real-world study., PMID:40047156
Results of personalized biological therapy in patients with chronic rhinosinusitis with nasal polyps and severe uncontrolled bronchial asthma - real-life study., PMID:40008473
Serum and urine eosinophil-derived neurotoxin (EDN) levels predict biologic response in severe asthma., PMID:39896201
Poor Agreement Among Asthma Specialists on the Choice and Timing of Initiation of a Biologic Treatment for Severe Asthma Patients., PMID:39864739
Use of Biologics to Treat Asthma during Pregnancy and Adverse Events in Pregnant Women and Newborns: A Global Pharmacovigilance Analysis., PMID:39827867
Impact of Smoking on Biological Treatment Response in Patients From the German Severe Asthma (GAN) Registry., PMID:39800060
Patient experience with eosinophilic esophagitis symptoms and impacts on daily life based on in-trial qualitative interviews., PMID:39777591
The Role of Bronchial Biopsy in the Prediction of Response to Biologic Therapy in Severe Uncontrolled Asthma: A Prospective Study., PMID:39742914
Imaging eosinophil secretory granules: From storage containers to active, immune responder organelles., PMID:39709031
Eosinophilic esophagitis., PMID:39702284
Caregiver worry about COVID-19 as a predictor of social mitigation behaviours and SARS-CoV-2 infection in a 12-city U.S. surveillance study of households with children., PMID:39697187
Single-cell RNA sequencing reveals transcriptional changes in circulating immune cells from patients with severe asthma induced by biologics., PMID:39672815
Biologics in allergology and clinical immunology: Update on therapies for atopic diseases, urticaria, and angioedema and on safety aspects focusing on hypersensitivity reactions., PMID:39600395
Off-Label Use of Monoclonal Antibodies for Eosinophilic Esophagitis in Humans: A Scoping Review., PMID:39595142
Drugs for asthma., PMID:39558440
Dupilumab Use in Patients With Hypereosinophilic Syndromes: A Multicenter Case Series and Review of the Literature., PMID:39515522
Disease Burden and Access to Biologic Therapy in Patients with Severe Asthma, 2017-2022: An Analysis of the International Severe Asthma Registry., PMID:39479509
Pharmacologic Treatment of Eosinophilic Esophagitis: Efficacious, Likely Efficacious, and Failed Drugs., PMID:39474328
Targeting the IL-5 pathway in eosinophilic asthma: A comparison of anti-IL-5 versus anti-IL-5 receptor agents., PMID:39396109
Real-World Studies of Biologics for the Treatment of Moderate-to-Severe Asthma., PMID:39389721
Asthma Biologics: Lung Function, Steroid-Dependence, and Exacerbations., PMID:39389718
Biologics in Hypereosinophilic Syndrome and Eosinophilic Granulomatosis with Polyangiitis., PMID:39389714
Disproportionality analysis of reslizumab based on the FDA Adverse Event Reporting System., PMID:39381062
Efficacy of Biologics in NSAID-ERD: United Airways From the Nose to the Bronchi., PMID:39343299
Up-dosing of reslizumab in severe asthmatics with persistent sputum eosinophilia: A feasibility study., PMID:39286951
Temporal variation in the effectiveness of biologics in asthma: Effect modification by changing patient characteristics., PMID:39260678
Choosing the Right Biologic for the Right Patient With Severe Asthma., PMID:39245321
A comparison of the impact of anti-IL5/5r therapies in allergic versus non-allergic patients with severe eosinophilic asthma in a real-life setting., PMID:39235972
One-food versus 4-food elimination diet for pediatric eosinophilic esophagitis: A multisite randomized trial., PMID:39233016
Amniotic fluid modifies esophageal epithelium differentiation and inflammatory responses., PMID:39189791
Therapeutic monoclonal antibodies in allergy: Targeting IgE, cytokine, and alarmin pathways., PMID:39158477
Induction of sustained remission and reversal of pathologic transcriptome achieved with tezepelumab in an adolescent with eosinophilic esophagitis., PMID:39134146
Comparative efficacy of biologics for patients with inadequately controlled asthma: A network meta-analysis., PMID:39091592
TH2 cell compensatory effect following benralizumab treatment for eosinophilic gastritis., PMID:39089335
Real-World Safety Profile of Biologic Drugs for Severe Uncontrolled Asthma: A Descriptive Analysis from the Spanish Pharmacovigilance Database., PMID:39064232
Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases., PMID:39062104
Losartan Treatment Reduces Esophageal Eosinophilic Inflammation in a Subset of Eosinophilic Esophagitis., PMID:39059581
Long-term durability between parent and child patient-reported outcomes in eosinophilic esophagitis., PMID:39059504