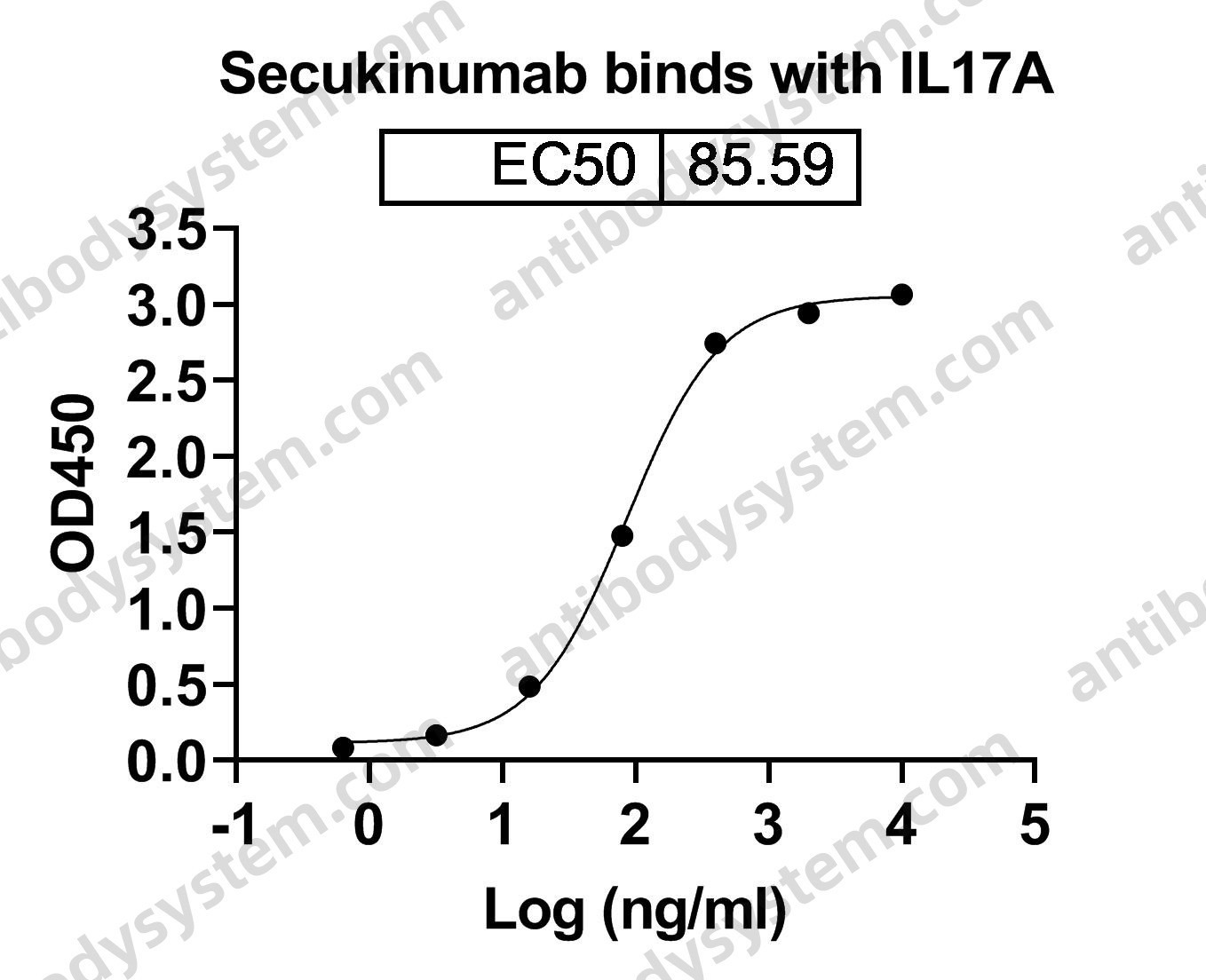
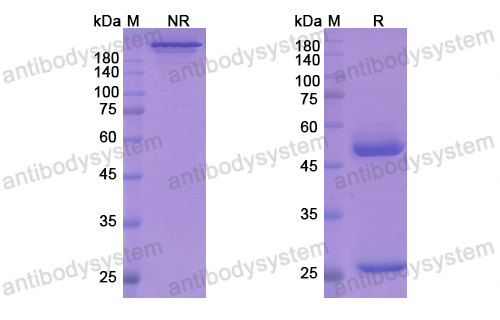
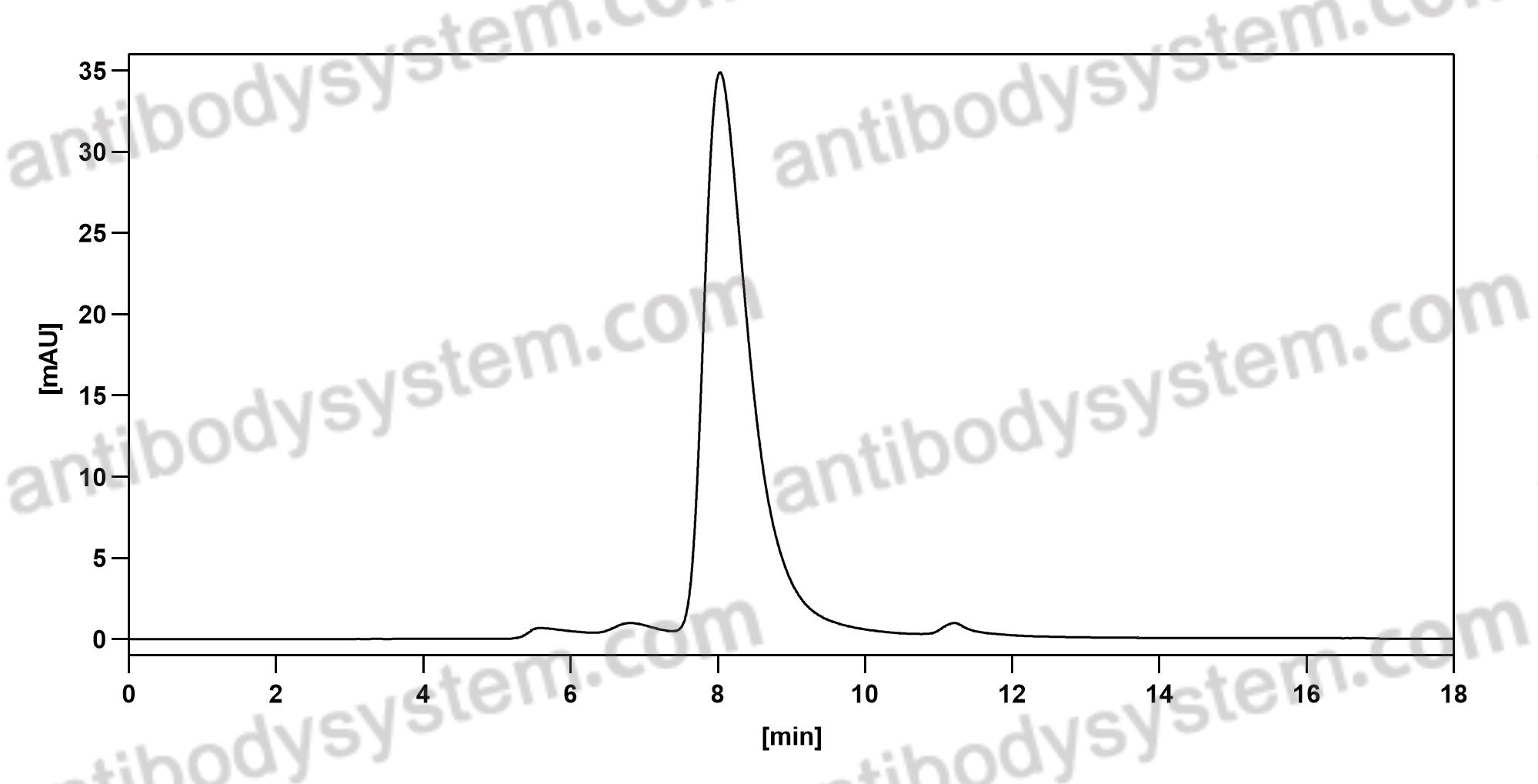
Secukinumab: A Review in Ankylosing Spondylitis, PMID: 30793255
Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial, PMID: 32386593
Safety of secukinumab in the treatment of psoriasis, PMID: 27545070
Secukinumab in plaque psoriasis--results of two phase 3 trials, PMID: 25007392
Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis, PMID: 26699169
Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial, PMID: 32594522
Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial, PMID: 31402114
Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis, PMID: 26422723
Secukinumab in the Treatment of Psoriasis and Psoriatic Arthritis: A Review of the Literature, PMID: 28732152
Improvement of Signs and Symptoms of Nonradiographic Axial Spondyloarthritis in Patients Treated With Secukinumab: Primary Results of a Randomized, Placebo-Controlled Phase III Study, PMID: 32770640
Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data, PMID: 31046809
Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial, PMID: 26135703
[Secukinumab], PMID: 31204124
Secukinumab for moderate-to-severe palmoplantar pustular psoriasis: Results of the 2PRECISE study, PMID: 30716404
Real-world evidence of secukinumab in psoriasis treatment - a meta-analysis of 43 studies, PMID: 31919937
Drug survival of secukinumab and ixekizumab for moderate-to-severe plaque psoriasis, PMID: 30914343
Comparative effectiveness of abatacept, apremilast, secukinumab and ustekinumab treatment of psoriatic arthritis: a systematic review and network meta-analysis, PMID: 29285605
Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study, PMID: 32479641
Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study), PMID: 29444376
Long-term efficacy and safety of secukinumab in the treatment of the multiple manifestations of psoriatic disease, PMID: 31785165
Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial, PMID: 26092291
Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients over 52 Weeks, PMID: 30508547
The advent of IL-17A blockade in ankylosing spondylitis: secukinumab, ixekizumab and beyond, PMID: 30576610
Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study, PMID: 30590813
Effects of secukinumab on metabolic and liver parameters in plaque psoriasis patients, PMID: 31599476
IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis, PMID: 31129129
Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study, PMID: 27663079
Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial, PMID: 33068444
IgA vasculitis during secukinumab therapy, PMID: 32860543
Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study, PMID: 31102257
Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR), PMID: 32124442
Secukinumab provided significant and sustained improvement in the signs and symptoms of ankylosing spondylitis: results from the 52-week, Phase III China-centric study, MEASURE 5, PMID: 32925287
Comparison of the Effects of Secukinumab and Adalimumab Biosimilar on Radiographic Progression in Patients with Ankylosing Spondylitis: Design of a Randomized, Phase IIIb Study (SURPASS), PMID: 31983056
Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY), PMID: 32365251
Safety of secukinumab for the treatment of active ankylosing spondylitis, PMID: 33470130
Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3, PMID: 29273067
Secukinumab in the treatment of psoriasis: an update, PMID: 28162025
Safety and effectiveness of secukinumab in psoriasis vulgaris and psoriatic arthritis: Real-world evidence in Japan, PMID: 33099791
Secukinumab provides rapid and persistent relief in pain and fatigue symptoms in patients with ankylosing spondylitis irrespective of baseline C-reactive protein levels or prior tumour necrosis factor inhibitor therapy: 2-year data from the MEASURE 2 study, PMID: 30148436
Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study, PMID: 31565244
Secukinumab-Induced Delayed-Type Drug Hypersensitivity Reactions, PMID: 31220050
Coronavirus disease 2019 (COVID-19) in a patient with ankylosing spondylitis treated with secukinumab: a case-based review, PMID: 32591970
Practical experience of secukinumab in the treatment of psoriasis: experience from a single centre, PMID: 32783092
Comparison of real-world treatment patterns among patients with psoriasis prescribed ixekizumab or secukinumab, PMID: 31712178
Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial, PMID: 30367462
Sustained efficacy of secukinumab in patients with moderate-to-severe palmoplantar psoriasis: 2·5-year results from GESTURE, a randomized, double-blind, placebo-controlled trial, PMID: 31286480
Secukinumab shows significant efficacy in palmoplantar psoriasis: Results from GESTURE, a randomized controlled trial, PMID: 27707593
Secukinumab (AIN457) for the treatment of psoriasis, PMID: 26428036
Secukinumab-associated localized granuloma annulare (SAGA): a case report and review of the literature, PMID: 32941717
Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3), PMID: 29544534
An update on the medical treatments of hidradenitis suppurativa., PMID:40530933
Neonatal CARD14-Associated Papulosquamous Eruption: Response to Secukinumab During Infancy., PMID:40525499
An evaluation of bimekizumab for the treatment of hidradenitis suppurativa., PMID:40524676
Disease Modification in Psoriasis Through Early IL-17 Inhibitor Intervention: A Retrospective Cohort Study., PMID:40516878
Dose modulation strategies in psoriatic patients: real-world comparison between secukinumab and brodalumab for up to one year after dose spacing., PMID:40499920
Deucravacitinib as a monotherapy for concurrent management of psoriasis and chronic spontaneous urticaria., PMID:40490621
Health Equity and Secukinumab for Severe Psoriasis in New Zealand., PMID:40488606
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
EXPRESS: THE FREQUENCY, PREDICTORS AND TREATMENT OPTIONS OF SPONDYLOARTHRITIS IN PRIMARY SJÖGREN'S SYNDROME: A SINGLE-CENTER EXPERIENCE., PMID:40488278
Uveitis in patients with axial spondyloarthritis or psoriatic arthritis: a post hoc analysis from placebo-controlled phase III studies with secukinumab., PMID:40487540
Effects of secukinumab on skeletal microarchitecture and vertebral fractures in patients with axial spondyloarthritis using HR-pQCT., PMID:40481972
Case Report: Effectiveness of deucravacitinib in chronic recurrent multifocal osteomyelitis and concomitant psoriasis., PMID:40475785
Physiologically Based Pharmacokinetic/Pharmacodynamic Model for Secukinumab: Dose Exploration in Pediatric Patients with Plaque Psoriasis., PMID:40464650
Safety and efficacy of IL-17 inhibitors in patients with comorbid multiple sclerosis/multiple Sclerosis-like syndrome: a systematic review., PMID:40459586
The effect of IL-17 and IL-23 ınhibitors on hematological ınflammatory parameters in patients with psoriasis vulgaris., PMID:40455345
IL-17A inhibitor-induced ulcerative colitis treated with an anti-IL-23 antibody., PMID:40455166
Model-Informed Drug Development-Based Bridging from Subcutaneous to Intravenous Secukinumab Dosing: Approval in Psoriatic Arthritis and Axial Spondyloarthritis., PMID:40454543
A Matching-Adjusted Indirect Comparison of Guselkumab and Secukinumab in Patients with Psoriatic Arthritis Over 52 Weeks., PMID:40450642
Case Report: Successful Treatment of Secukinumab-Induced SAPHO Syndrome With Tofacitinib., PMID:40443046
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Clinical Characteristics of CARD14-Associated Papulosquamous Eruption and Evaluation of Therapeutic Efficacy of Secukinumab., PMID:40433052
Evaluation of combination therapy with secukinumab and systemic corticosteroids for acute generalized exanthematous pustulosis: A retrospective cohort study., PMID:40419223
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Causes of drug-induced photosensitivity: an analysis using FDA adverse event reporting system database., PMID:40413262
Real-World Utilization of Biologic and Targeted Synthetic Disease-Modifying Anti-rheumatic Drugs in Psoriatic Arthritis and Axial Spondyloarthritis: Insights from Sweden and Germany., PMID:40402376
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Biologic and Non-Biologic Therapies for Scalp Psoriasis: A Network Meta-analysis of Randomized Controlled Trials., PMID:40401874
Safety and Efficacy of Secukinumab in a Child with CARD14-Associated Papulosquamous Eruption: A Case Report and Review of Literature., PMID:40395577
Systemic secukinumab treatment in patients with ankylosing spondylitis: the relationship between systemic and tear proinflammatory cytokines and ocular surface findings-a pilot study., PMID:40394377
Bayesian trial of adalimumab versus secukinumab for children with juvenile idiopathic arthritis associated uveitis or chronic anterior uveitis., PMID:40390069
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Access, Effectiveness, Safety, and Survival of Secukinumab in Patients With Axial Spondyloarthritis and Axial Psoriatic Arthritis: A Real-World Study in Argentina., PMID:40374511
Biologic therapies and small molecules in the treatment of hidradenitis suppurativa., PMID:40373949
Assessing Long-Term Pain Reduction with Secukinumab in Moderate to Severe Hidradenitis Suppurativa: A Post Hoc Analysis of the SUNSHINE and SUNRISE Phase 3 Trials., PMID:40372667
Extension of Secukinumab and Ixekizumab Dose for Moderate-To-Severe Psoriasis in Low Disease Activity Intervals., PMID:40369849
Secukinumab is effective and safe for patients with giant cell arteritis after tocilizumab failure., PMID:40366735
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Combined biological therapy with dupilumab and secukinumab for coexisting bullous pemphigoid and psoriasis., PMID:40357969
Secukinumab induced paradoxical worsening of mucocutaneous features of Behcet's disease., PMID:40357952
Apremilast Coadministered with Secukinumab for Effective Treatment of Acrodermatitis Continua of Hallopeau: A Case Report., PMID:40351850
Hypoxia-induced RHCG as a key regulator in psoriasis and its modulation by secukinumab., PMID:40351602
First case report of oral histoplasmosis in a patient receiving Secukinumab (Cosentyx): opportunistic infections as complication of emerging immunomodulatory therapies., PMID:40350329
Treatment of severe psoriasis in a hospice care patient using secukinumab, an inhibitor of interleukin-17A expression: Treatment response and changes in quality of life., PMID:40329840
Aggregate Distributional Cost-Effectiveness Analysis of Biologics for the Treatment of Ankylosing Spondylitis in Chile., PMID:40329067
Emerging manifestations of IL-17 immunomodulation in the gastrointestinal tract., PMID:40319948
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Letter to the Editor for "Which Is the Best Option for AxSpA Patients After First TNFi Failure: Switch to Secukinumab or Cycling With Other TNFi?"., PMID:40312866
Secukinumab Leading to Rapid Improvement in Pyogenic Arthritis, Acne, Pyoderma Gangrenosum, and Hidradenitis Suppurativa (PAPASH) Syndrome: A Case Report and Review of Treatment Modalities for PAPASH Patients., PMID:40309341