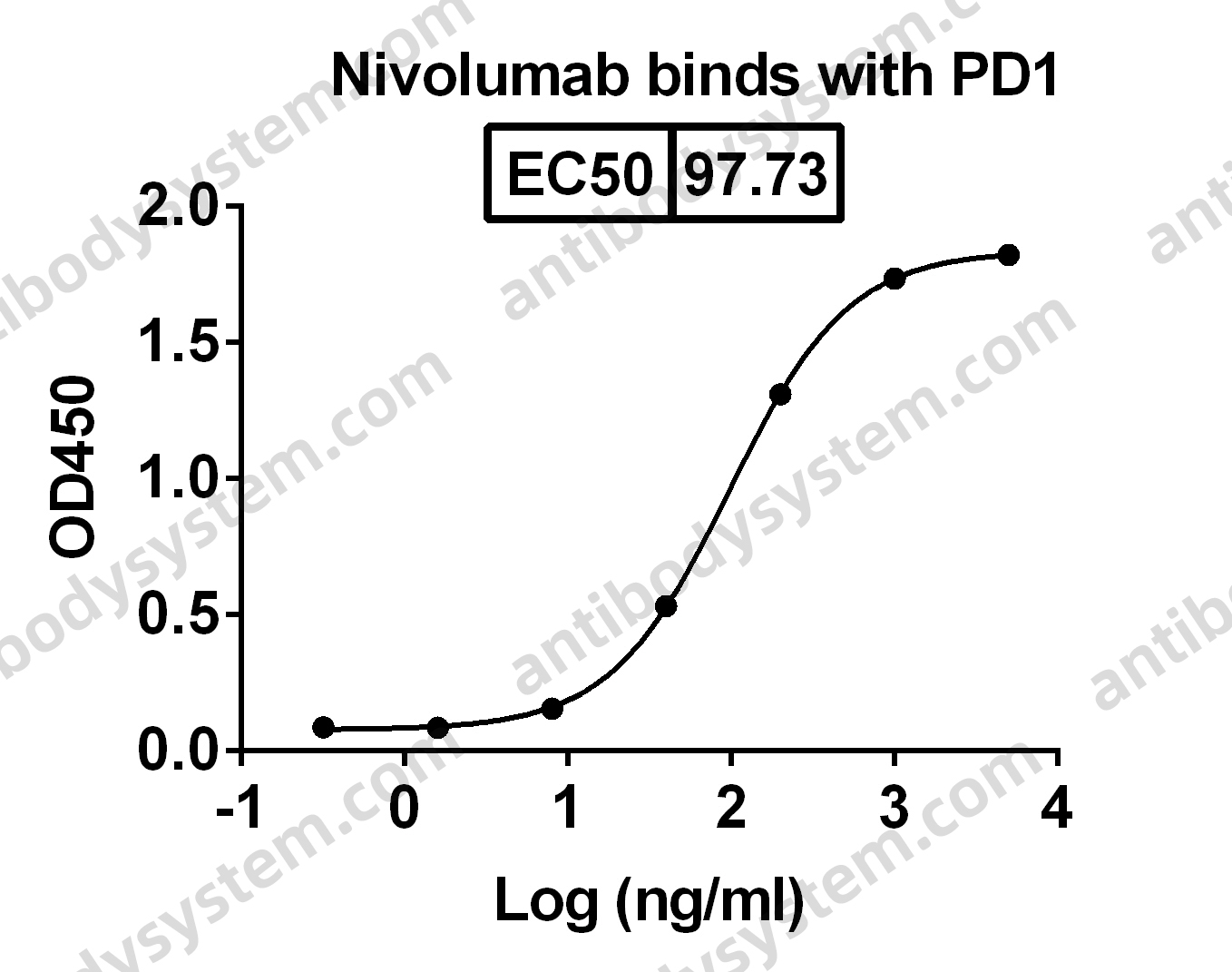
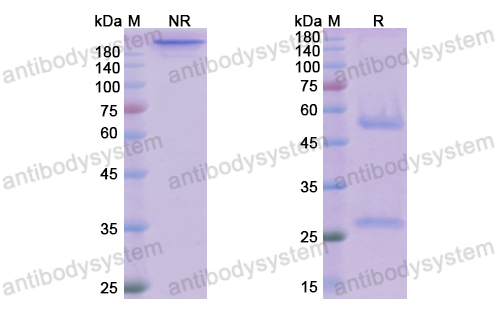
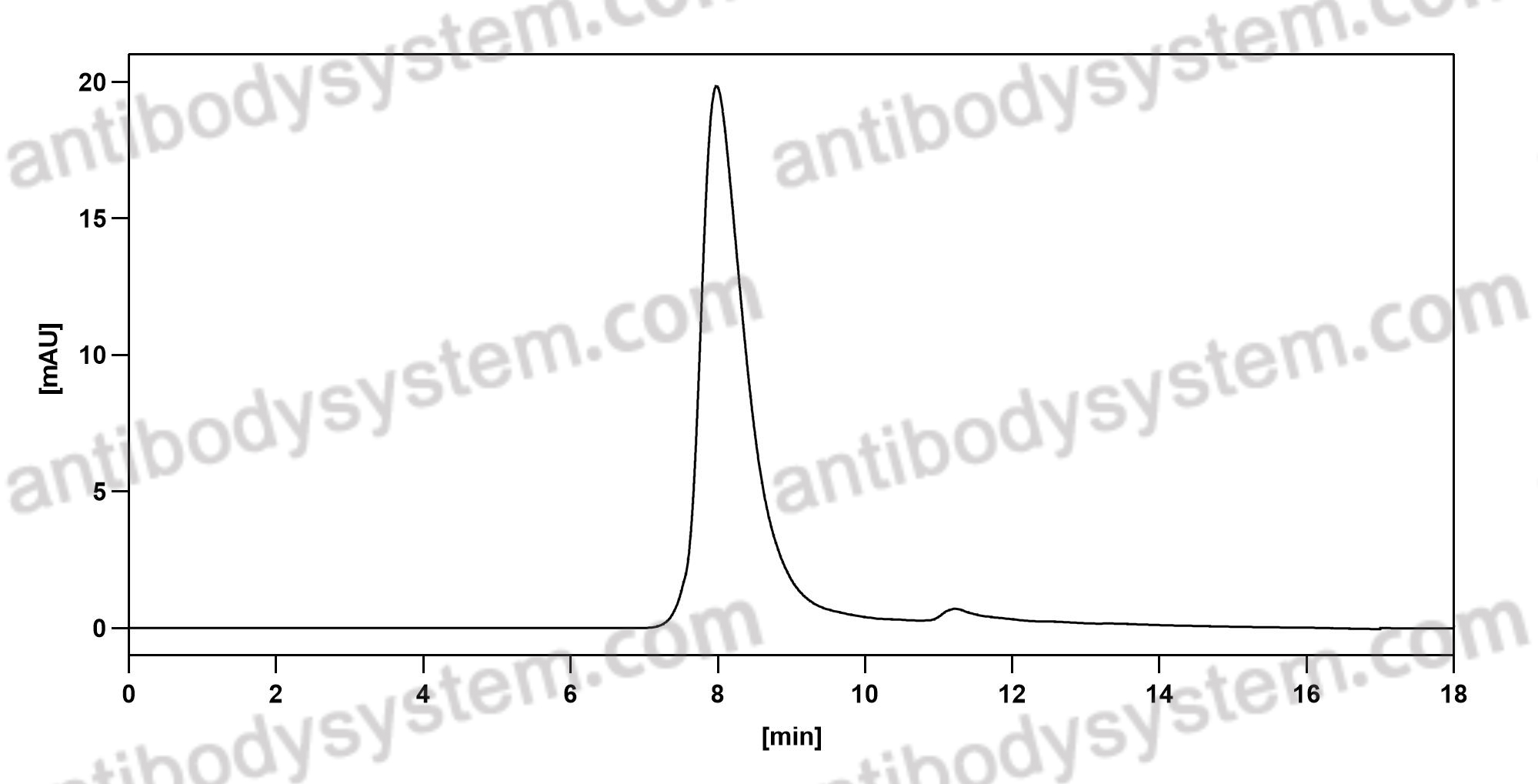
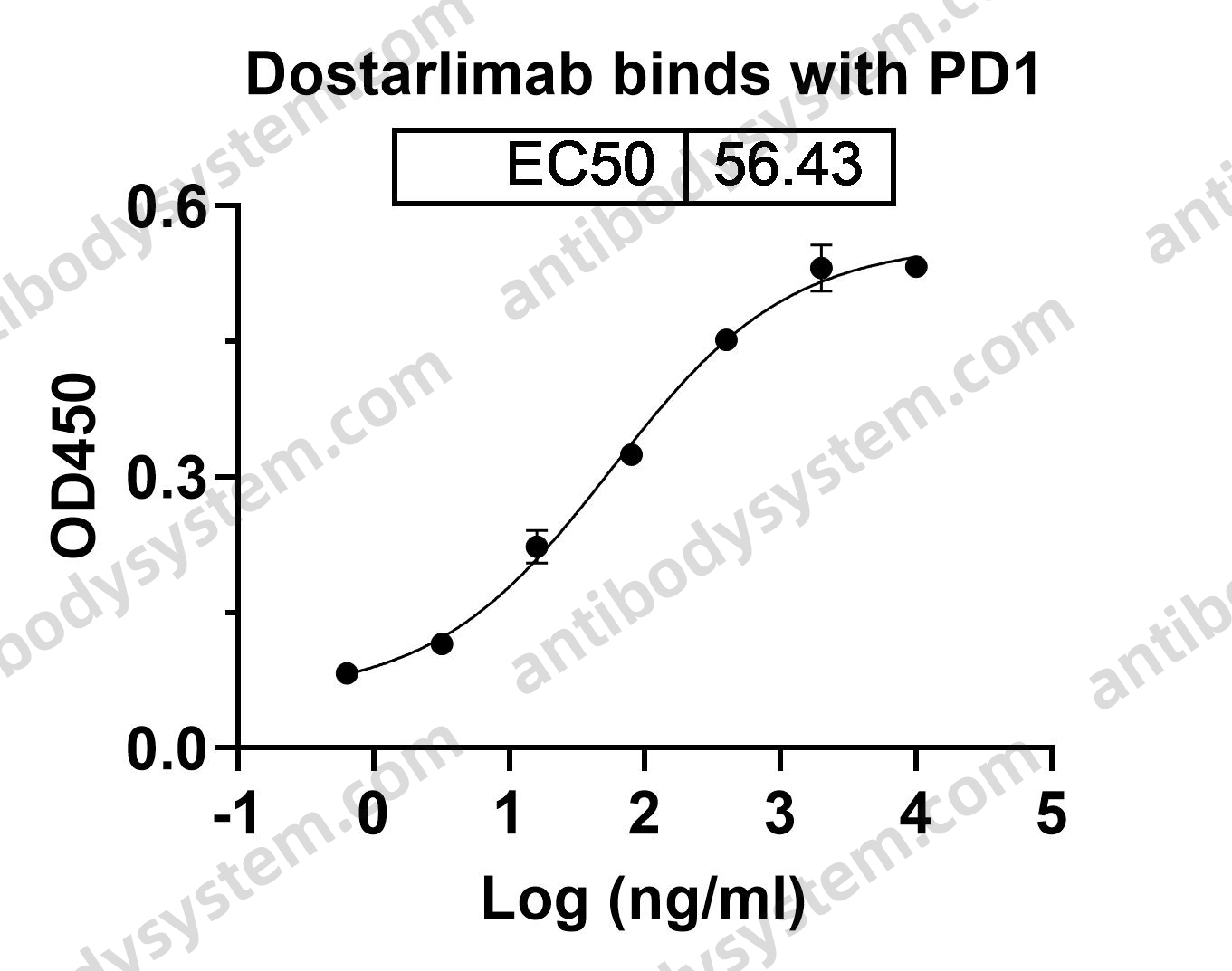
Nivolumab for the treatment of colorectal cancer, PMID: 29792730
Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer, PMID: 29355075
Nivolumab for the treatment of hepatocellular carcinoma, PMID: 30304963
Nivolumab for the treatment of hepatocellular carcinoma, PMID: 32249635
Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression, PMID: 29884413
Checkpoint immunotherapy by nivolumab for treatment of metastatic melanoma, PMID: 30488824
Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort, PMID: 31629915
Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma, PMID: 30124333
Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial, PMID: 32673417
Continuous Versus 1-Year Fixed-Duration Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: CheckMate 153, PMID: 32910710
Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial, PMID: 30659987
Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience, PMID: 32507911
Cost-effectiveness of nivolumab in advanced melanoma: a drug review, PMID: 33225752
Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults, PMID: 31914172
An update on the safety of nivolumab for the treatment of advanced melanoma, PMID: 32293935
Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study, PMID: 29570421
Nivolumab for the treatment of urothelial cancers, PMID: 29363363
Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial, PMID: 32852531
Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma, PMID: 32822275
Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC, PMID: 29802888
Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study, PMID: 31112476
Nivolumab: an investigational agent for the treatment of biliary tract cancer, PMID: 33307866
Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer, PMID: 30215677
Nephrotoxicity Associated with Novel Anticancer Agents (Aflibercept, Dasatinib, Nivolumab): Case Series and Nephrological Considerations, PMID: 32664269
Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078), PMID: 33321441
Nivolumab in squamous cell carcinoma of the head and neck, PMID: 29560755
Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab, PMID: 31638948
Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: A multicenter, open-label single-arm phase II trial, PMID: 31182249
CheckMate 141: 1-Year Update and Subgroup Analysis of Nivolumab as First-Line Therapy in Patients with Recurrent/Metastatic Head and Neck Cancer, PMID: 29866947
Nivolumab-induced lichen planus, PMID: 31382865
Effectiveness and safety of nivolumab in patients with head and neck cancer in Japanese real-world clinical practice: a multicenter retrospective clinical study, PMID: 33219460
Nivolumab for Treating Metastatic or Unresectable Urothelial Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal, PMID: 30293207
Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma, PMID: 29908324
Nivolumab-induced erosive pustular dermatosis of the scalp, PMID: 32516431
Nivolumab-induced toxic epidermal necrolysis with retiform purpura, PMID: 32281097
Nivolumab-Induced Periaortitis Demonstrated by FDG PET/CT, PMID: 32701815
Nivolumab for the Treatment of Advanced Pediatric Malignancies, PMID: 33288608
Activity of regorafenib plus nivolumab, PMID: 32398704
Two-year follow-up of a randomized phase III clinical trial of nivolumab vs. the investigator's choice of therapy in the Asian population for recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141), PMID: 32583557
Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non-Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153), PMID: 31121324
Rapid onset type-1 diabetes and diabetic ketoacidosis secondary to nivolumab immunotherapy: a review of existing literature, PMID: 31451458
Nivolumab-related severe thrombocytopenia in a patient with relapsed lung adenocarcinoma: a case report and review of the literature, PMID: 31647029
Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study, PMID: 30642819
Progression-Free and Overall Survival for Concurrent Nivolumab With Standard Concurrent Chemoradiotherapy in Locally Advanced Stage IIIA-B NSCLC: Results From the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6-14), PMID: 33188912
Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types, PMID: 31959348
Nivolumab plus gemcitabine, dexamethasone, and cisplatin chemotherapy induce durable complete remission in relapsed/refractory primary mediastinal B-cell lymphoma: a case report and literature review, PMID: 32783492
Association of Immune-Related Adverse Events with Clinical Benefit in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab, PMID: 29934411
[Nivolumab-induced hypothyroidism: A case report], PMID: 30837393
Dermatomyositis associated with nivolumab therapy for melanoma: a case report and review of the literature, PMID: 32941716
Agnostic evaluation of ipilimumab and nivolumab association: a metanalysis, PMID: 33239030
An evaluation of Nivolumab and ipilimumab for the treatment of resectable stage III melanoma., PMID:40531544
Case Report: Development of severe inflammatory orbitopathy after immune checkpoint inhibitor initiation., PMID:40529668
Pseudomembranous Colitis: Unveiling an Infrequent Culprit Beyond Clostridium difficile-A Case Report., PMID:40528805
Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (PE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161., PMID:40526078
Treatment Effect Estimation With Potential Outcomes for a Single-Arm Trial Compared With Historical Controls: A Case Study of Locally Recurrent Head and Neck Squamous Cell Carcinoma Patients Treated With Adjuvant Nivolumab., PMID:40525328
Nivolumab-AVD Versus Brentuximab Vedotin-AVD in Older Patients With Advanced-Stage Classic Hodgkin Lymphoma Enrolled on S1826., PMID:40523203
Rapid-Onset Myositis and Myocarditis Following Dual Immune Checkpoint Inhibitor Therapy With Nivolumab and Ipilimumab for Gastric Adenocarcinoma: A Case Report., PMID:40519473
Uncovering prognostic biomarkers through a pharmacovigilance study: the case of RDW., PMID:40517345
Phase II study of rucaparib and nivolumab in patients with leiomyosarcoma., PMID:40514070
Time toxicity of nivolumab in metastatic head and neck squamous cell carcinoma patients: a single-institution experience., PMID:40514043
Expanding access to cancer immunotherapy: A systematic review of low-dose PD-(L)1 inhibitor strategies., PMID:40513286
Nivolumab plus relatlimab in advanced melanoma: RELATIVITY-047 4-year update., PMID:40513285
Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis., PMID:40507352
Immune Checkpoint Inhibitors in the Treatment of Advanced Melanoma in Older Patients: An Overview of Published Data., PMID:40507314
Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy., PMID:40507305
Baseline Radiomics as a Prognostic Tool for Clinical Benefit from Immune Checkpoint Inhibition in Inoperable NSCLC Without Activating Mutations., PMID:40507271
Tight competition for the first line treatment of Hodgkin's lymphoma: a comprehensive review of practice-changing developments in recent years., PMID:40506833
Neoadjuvant nivolumab plus chemotherapy improves overall survival in resectable NSCLC., PMID:40506587
Combined immunotherapy with nivolumab and ipilimumab with and without sequential or concomitant stereotactic radiotherapy in patients with melanoma brain metastasis: An international retrospective study., PMID:40505525
Treatment of older patients with Hodgkin lymphoma., PMID:40504313
Case Report: Locally invasive thyroid metastases from renal cell carcinoma: surgery after neoadjuvant therapy., PMID:40502628
NEXUS: a phase I dose escalation study of selinexor plus nivolumab and ipilimumab in Asian patients with advanced/metastatic solid malignancies., PMID:40502306
Engraftment Syndrome Following Autologous Stem Cell Transplantation in a Patient Receiving Nivolumab as Salvage Therapy., PMID:40497705
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
Enfortumab vedotin induced interstitial lung disease: A first case report with pathological evidence from transbronchial lung cryobiopsy., PMID:40496844
Drug-associated abdominal aortitis and retroperitoneal fibrosis after treatment with nivolumab., PMID:40496660
The real-world safety of Nivolumab: a pharmacovigilance analysis based on the FDA adverse event reporting system., PMID:40491923
Clinical Outcomes in Patients With Muscle-Invasive Urothelial Carcinoma Treated With Nivolumab., PMID:40489108
Biomarker analysis and treatment dynamics following preoperative ipilimumab plus nivolumab in locally advanced urothelial cancer from the phase 1B NABUCCO study., PMID:40489082
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
Organ-Specific Responses to Nivolumab Plus Ipilimumab in Advanced Hepatocellular Carcinoma: A Multicenter, Retrospective Study., PMID:40488227
Signet Ring Cell Carcinoma of the Stomach with Chylous Pleural Effusion: Laterality Shift and Fluid Composition Change., PMID:40487559
Safety and short-term outcomes of adjuvant nivolumab therapy at 480 mg dose every four weeks in resected esophageal cancer: a prospective cohort study., PMID:40484886
Cost-Utility and Budget Impact Analysis of Immunotherapy for First-Line Treatment of Advanced Kidney Cancer in Latin America: Evidence From Uruguay., PMID:40482312
Validation of a 15-Gene Prognostic Signature in Metastatic Clear Cell Renal Cell Carcinoma., PMID:40479621
A multicenter Phase II study of mFOLFOX6 plus nivolumab for gastric cancer with severe peritoneal metastases: WJOG16322G., PMID:40476844
"Attenuated" Pulmonary Tumor Thrombotic Microangiopathy on Anti-Vascular Endothelial Growth Factor Treatment: A Case Report., PMID:40475293
A phase II study of nivolumab, ipilimumab, and radiation in combination with influenza vaccine in patients with pancreatic cancer (INFLUENCE)., PMID:40475164
Immune checkpoint inhibitor therapy in metastatic renal cell carcinoma: tumour response and immune-related renal vasculitis following cytoreductive nephrectomy., PMID:40474803
Cell-mediated mucositis of the oral cavity: narrative review on etiology, clinico-pathological aspects and malignant transformation., PMID:40474707
Inherited mitochondrial genetics as a predictor of immune checkpoint inhibition efficacy in melanoma., PMID:40473950
Assessing individual head and neck squamous cell carcinoma patient response to therapy through integration of functional and genomic data., PMID:40473660
Treatment-Free Survival Over 6 Years of Follow-up in Patients With Metastatic Non-small Cell Lung Cancer Treated With First-Line Nivolumab Plus Ipilimumab Versus Chemotherapy in CheckMate 227 Part 1., PMID:40473108
Phase II trial dedicated to non-selected, pretreated cutaneous angiosarcoma: Efficacy of nivolumab (AngioCheck Study)., PMID:40472568
Nivolumab Induces Durable Remission in Relapsed/Refractory ALK-Negative ALCL After Allogeneic Blood Stem Cell Transplantation-A Case Report and Literature Review., PMID:40469417
Risk factors for checkpoint inhibitor pneumonitis in lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis., PMID:40469302
Peripheral memory B cell population maintenance and long-term survival after perioperative chemoimmunotherapy in NSCLC (NADIM trial)., PMID:40468805
Immune-mediated enterocolitis is associated with immune checkpoint inhibitors: A pharmacovigilance study from the FDA Adverse Event Reporting System (FAERS) database., PMID:40465755
Successful use of nivolumab and relatlimab as salvage therapy for metastatic uveal melanoma., PMID:40465233
Impact of medications on the efficacy of immune checkpoint inhibitors in patients with recurrent or metastatic head and neck cancer., PMID:40465127