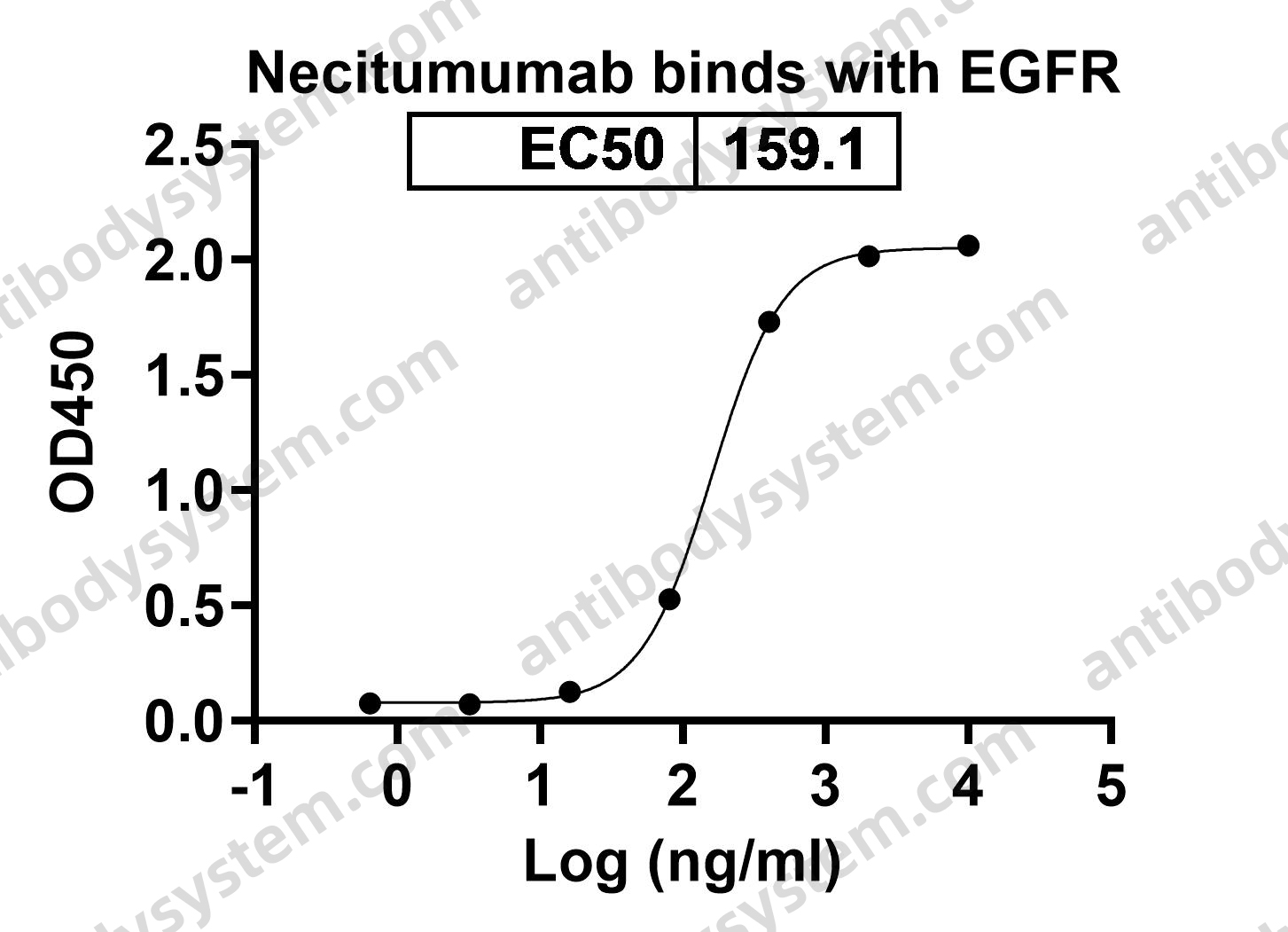
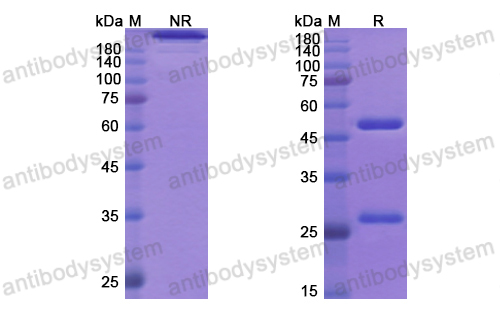
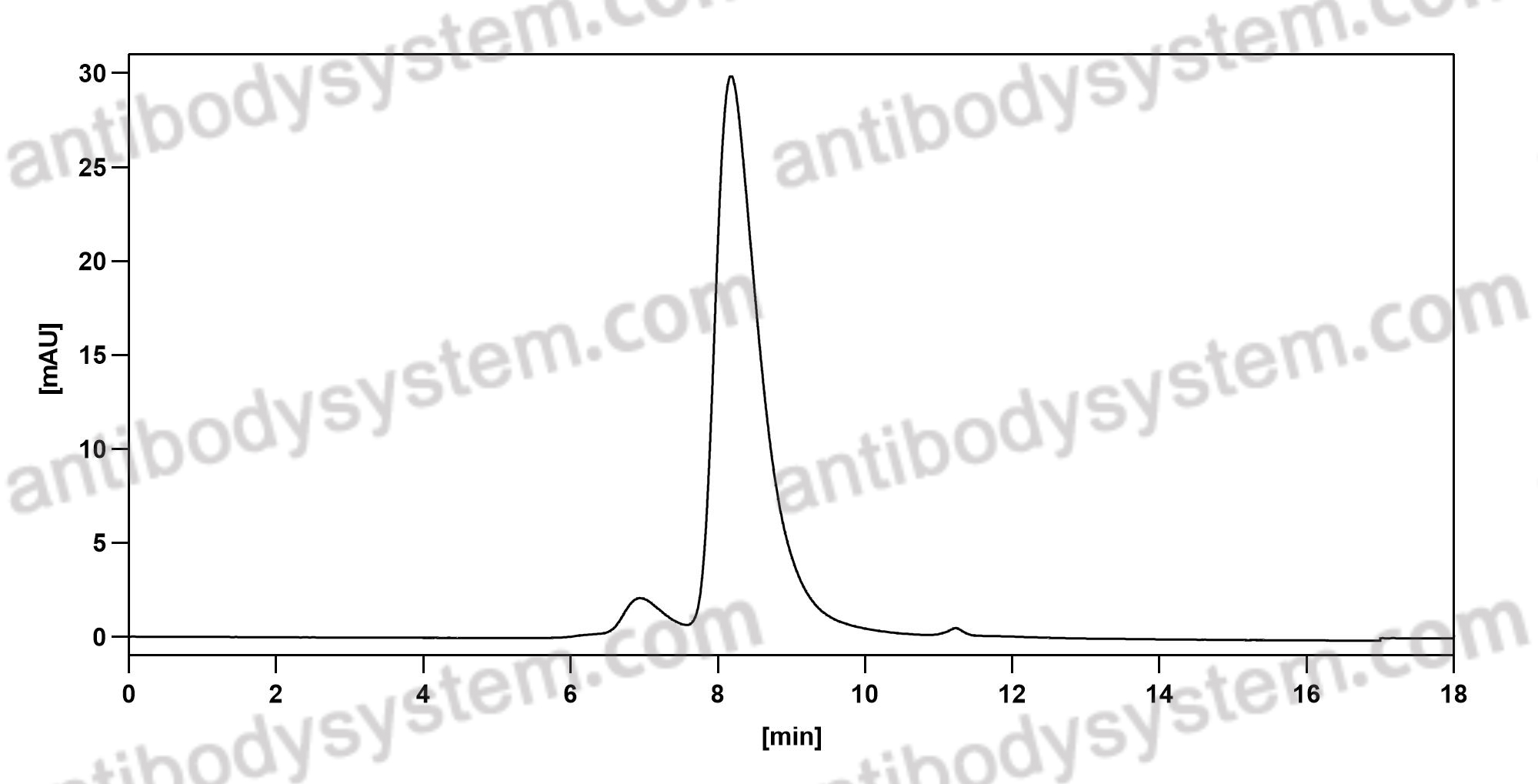
Necitumumab, PMID: 31643800
Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial, PMID: 26045340
Necitumumab, PMID: 29999841
Necitumumab for the treatment of advanced non-small-cell lung cancer, PMID: 30501503
Necitumumab in the treatment of non-small-cell lung cancer: clinical controversies, PMID: 30075697
Necitumumab: First Global Approval, PMID: 26729188
Necitumumab: a new option for first-line treatment of squamous cell lung cancer, PMID: 30025476
Necitumumab, a fully human IgG1 mAb directed against the EGFR for the potential treatment of cancer, PMID: 21154125
Necitumumab for non-small cell lung cancer, PMID: 26051700
Necitumumab for the treatment of squamous cell non-small cell lung cancer, PMID: 27913776
Spotlight on necitumumab in the treatment of non-small-cell lung carcinoma, PMID: 28293124
Clinical potential of necitumumab in non-small cell lung carcinoma, PMID: 27621656
Necitumumab in Metastatic Squamous Cell Lung Cancer: Establishing a Value-Based Cost, PMID: 26313558
Necitumumab in the treatment of advanced non-small cell lung cancer: translation from preclinical to clinical development, PMID: 21679088
Population Pharmacokinetics of Necitumumab in Cancer Patients, PMID: 27696220
Necitumumab plus platinum-based chemotherapy versus chemotherapy alone as first-line treatment for stage IV non-small cell lung cancer: a meta-analysis based on randomized controlled trials, PMID: 32954751
Will Necitumumab Be Cost-Effective?, PMID: 26380602
Efficacy and safety of necitumumab and pembrolizumab combination therapy in patients with Stage IV non-small cell lung cancer, PMID: 32114283
Molecular Basis for Necitumumab Inhibition of EGFR Variants Associated with Acquired Cetuximab Resistance, PMID: 29158469
The Effect of Necitumumab in Combination with Gemcitabine plus Cisplatin on Tolerability and on Quality of Life: Results from the Phase 3 SQUIRE Trial, PMID: 26980471
Effects of adding necitumumab to first-line chemotherapy in patients with stage IV non-small-cell lung cancer: Meta-analysis, PMID: 31822198
A phase 1b study of necitumumab in combination with abemaciclib in patients with stage IV non-small cell lung cancer, PMID: 31586771
A phase II study of nab-paclitaxel and carboplatin chemotherapy plus necitumumab in the first-line treatment of patients with stage IV squamous non-small cell lung cancer, PMID: 31445354
Efficacy and Safety of Necitumumab Continuation Therapy in the Phase III SQUIRE Study of Patients With Stage IV Squamous Non-Small-Cell Lung Cancer, PMID: 29158123
Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study, PMID: 25701171
Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous non-small cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan, PMID: 30797492
Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer, PMID: 29175116
Necitumumab in squamous non-small-cell lung cancer: how to move forward?, PMID: 27329250
Evaluation of the effect of necitumumab on the corrected QT interval in patients with advanced solid tumors, PMID: 27312733
Necitumumab plus Gemcitabine and Cisplatin as First-Line Therapy in Patients with Stage IV EGFR- Expressing Squamous Non-Small-Cell Lung Cancer: German Subgroup Data from an Open-Label, Randomized Controlled Phase 3 Study (SQUIRE), PMID: 27614872
A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies, PMID: 20197484
Exposure-Response Analysis of Necitumumab Efficacy in Squamous Non-Small Cell Lung Cancer Patients, PMID: 28569042
Drug Monographs: Ixazomib and Necitumumab, PMID: 27303088
Necitumumab for patients with non-squamous NSCLC: uninspiring results, PMID: 25701169
Necitumumab for the treatment of stage IV metastatic squamous non-small-cell lung cancer, PMID: 25797462
Cangrelor, PMID: 27729681
EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles, PMID: 33188892
EGFR Gene Copy Number by FISH May Predict Outcome of Necitumumab in Squamous Lung Carcinomas: Analysis from the SQUIRE Study, PMID: 29158193
An Open-Label, Randomized, Controlled Phase II Study of Paclitaxel-Carboplatin Chemotherapy With Necitumumab Versus Paclitaxel-Carboplatin Alone in First-Line Treatment of Patients With Stage IV Squamous Non-Small-Cell Lung Cancer, PMID: 28365238
Correlation of EGFR-expression with safety and efficacy outcomes in SQUIRE: a randomized, multicenter, open-label, phase III study of gemcitabine-cisplatin plus necitumumab versus gemcitabine-cisplatin alone in the first-line treatment of patients with stage IV squamous non-small-cell lung cancer, PMID: 27207107
Corrigendum to "Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous non-small cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan" [Lung Cancer, 129 (March) (2019) 55-62], PMID: 31014853
Necitumumab: a new therapeutic option for squamous cell lung cancer?, PMID: 25806327
Portrazza (Necitumumab), an IgG1 Monoclonal Antibody, FDA Approved for Advanced Squamous Non-Small-Cell Lung Cancer, PMID: 27668058
Necitumumab for first-line treatment of advanced, squamous, non-small-cell lung cancer: a relevant step forward?, PMID: 26958500
Efficacy and Safety of First-Line Necitumumab Plus Gemcitabine and Cisplatin Versus Gemcitabine and Cisplatin in East Asian Patients with Stage IV Squamous Non-small Cell Lung Cancer: A Subgroup Analysis of the Phase 3, Open-Label, Randomized SQUIRE Study, PMID: 28111429
NICE guidance on necitumumab for untreated advanced or metastatic squamous non-small-cell lung cancer, PMID: 27692477
Phase II study of necitumumab plus modified FOLFOX6 as first-line treatment in patients with locally advanced or metastatic colorectal cancer, PMID: 26766738
Venous thromboembolism with EGFR monoclonal antibody necitumumab in stage IV non-small cell lung cancer: A retrospective cohort analysis, PMID: 29787943
Phase I study of the second-generation, recombinant, human EGFR antibody necitumumab in Japanese patients with advanced solid tumors, PMID: 27628194
The Budget Impact of Including Necitumumab on the Formulary for First-Line Treatment of Metastatic Squamous Non-Small Cell Lung Cancer: U.S. Commercial Payer and Medicare Perspectives, PMID: 29799326
ORCHARD: Osimertinib Plus Necitumumab in Patients With Epidermal Growth Factor Receptor-Mutated Advanced Non-Small Cell Lung Cancer With a Secondary Epidermal Growth Factor Receptor Alteration Whose Disease Had Progressed on First-Line Osimertinib., PMID:40466026
Skin disorder within 30 days is a favorable prognostic factor in patients with lung squamous cell carcinoma treated with necitumumab plus gemcitabine and cisplatin: a sub-analysis of the NINJA study., PMID:40093977
A Case of EGFR-mutant Squamous Cell Lung Cancer Treated With Necitumumab Combination Therapy., PMID:40010994
Clinical impact of hypomagnesemia induced by necitumumab plus cisplatin and gemcitabine treatment in patients with advanced lung squamous cell carcinoma: a subanalysis of the NINJA study., PMID:39957806
Afatinib and Necitumumab in EGFR-Mutant NSCLC with Acquired Resistance to Tyrosine Kinase Inhibitors., PMID:39866193
A Case of Advanced Biliary Tract Cancer With EGFR Amplification That Responded to Necitumumab., PMID:39540676
Transforming Lung Cancer Management: A Promising Case Study of Immune Checkpoint Inhibitor Success Following a Multidisciplinary Approach., PMID:39410563
Effects of N361 Glycosylation on Epidermal Growth Factor Receptor Biological Function., PMID:39071333
Enhancing Neutrophil Cytotoxicity of a Panel of Clinical EGFR Antibodies by Fc Engineering to IgA3.0., PMID:38958494
Association between skin toxicity and efficacy of necitumumab in squamous non-small-cell lung cancer: a pooled analysis of two randomized clinical trials-SQUIRE and JFCM., PMID:38520847
[Enhancement of Antitumor Effect by the Combined Use of Anti-EGFR Antibody(Necitumumab)and PD-1 Inhibitor]., PMID:38247069
Multicenter, Retrospective Study to Evaluate Necitumumab Plus Cisplatin and Gemcitabine After Immune Checkpoint Inhibitors in Advanced Squamous Cell Lung Cancer in Japan: The NINJA Study., PMID:38046378
A Phase I/II Study of Necitumumab Plus Pembrolizumab, Nab-Paclitaxel, and Carboplatin for Previously Untreated Advanced Squamous Non-Small Cell Lung Cancer Study: (NEJ048A/NEXUS)., PMID:36849264
Necitumumab plus gemcitabine and cisplatin in previously treated lung squamous cell carcinoma., PMID:36331673
Acquired perforating dermatosis induced by necitumumab., PMID:35686644
Treatment Sequencing Strategies in Lung Cancer., PMID:35599008
Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases., PMID:35458666
Objective Quantitation of EGFR Protein Levels using Quantitative Dot Blot Method for the Prognosis of Gastric Cancer Patients., PMID:35079437
Accurate determination of epitope for antibodies with unknown 3D structures., PMID:34432559
Lung squamous cell carcinoma with severe hypomagnesemia due to cisplatin plus gemcitabine in combination with necitumumab therapy: A case report., PMID:34061460
EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles., PMID:33188892
Necitumumab plus platinum-based chemotherapy versus chemotherapy alone as first-line treatment for stage IV non-small cell lung cancer: a meta-analysis based on randomized controlled trials., PMID:32954751
The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells., PMID:32793499
Efficacy and safety of necitumumab and pembrolizumab combination therapy in patients with Stage IV non-small cell lung cancer., PMID:32114283
Effects of adding necitumumab to first-line chemotherapy in patients with stage IV non-small-cell lung cancer: Meta-analysis., PMID:31822198
A phase 1b study of necitumumab in combination with abemaciclib in patients with stage IV non-small cell lung cancer., PMID:31586771
A phase II study of nab-paclitaxel and carboplatin chemotherapy plus necitumumab in the first-line treatment of patients with stage IV squamous non-small cell lung cancer., PMID:31445354
The effects of somatic mutations on EGFR interaction with anti-EGFR monoclonal antibodies: Implication for acquired resistance., PMID:31228284
Corrigendum to "Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous non-small cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan" [Lung Cancer, 129 (March) (2019) 55-62]., PMID:31014853
Most clinical anti-EGFR antibodies do not neutralize both wtEGFR and EGFRvIII activation in glioma., PMID:31002307
Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous non-small cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan., PMID:30797492
First-line treatment of patients with advanced or metastatic squamous non-small cell lung cancer: systematic review and network meta-analysis., PMID:30746213
The people behind the papers - Elena Popa and Abigail Tucker., PMID:30737241
Necitumumab for the treatment of advanced non-small-cell lung cancer., PMID:30501503
Immune Effector Functions of Human IgG2 Antibodies against EGFR., PMID:30282813
Necitumumab in the treatment of non-small-cell lung cancer: clinical controversies., PMID:30075697
Necitumumab: a new option for first-line treatment of squamous cell lung cancer., PMID:30025476
Algorithm for the treatment of advanced or metastatic squamous non-small-cell lung cancer: an evidence-based overview., PMID:29910650
Predictive biomarkers for response to EGFR-directed monoclonal antibodies for advanced squamous cell lung cancer., PMID:29905778
Breakthroughs in the treatment of advanced squamous-cell NSCLC: not the neglected sibling anymore?, PMID:29862232
The Budget Impact of Including Necitumumab on the Formulary for First-Line Treatment of Metastatic Squamous Non-Small Cell Lung Cancer: U.S. Commercial Payer and Medicare Perspectives., PMID:29799326
Venous thromboembolism with EGFR monoclonal antibody necitumumab in stage IV non-small cell lung cancer: A retrospective cohort analysis., PMID:29787943
A retrospective examination of the US Food and Drug Administration's clinical pharmacology reviews of oncology biologics for potential use of therapeutic drug monitoring., PMID:29343970
Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer., PMID:29175116
Molecular Basis for Necitumumab Inhibition of EGFR Variants Associated with Acquired Cetuximab Resistance., PMID:29158469
EGFR Gene Copy Number by FISH May Predict Outcome of Necitumumab in Squamous Lung Carcinomas: Analysis from the SQUIRE Study., PMID:29158193
Efficacy and Safety of Necitumumab Continuation Therapy in the Phase III SQUIRE Study of Patients With Stage IV Squamous Non-Small-Cell Lung Cancer., PMID:29158123
EGFR immunohistochemistry as biomarker for antibody-based therapy of squamous NSCLC - Experience from the first ring trial of the German Quality Assurance Initiative for Pathology (QuIP®)., PMID:29108919
Meta-analysis of first-line therapies with maintenance regimens for advanced non-small-cell lung cancer (NSCLC) in molecularly and clinically selected populations., PMID:28675660