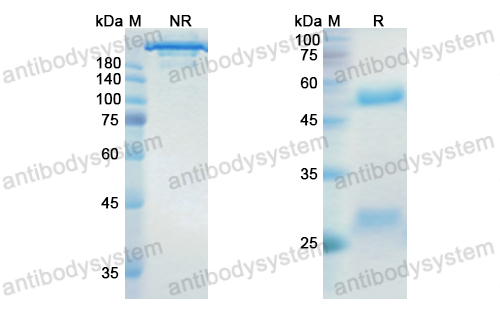
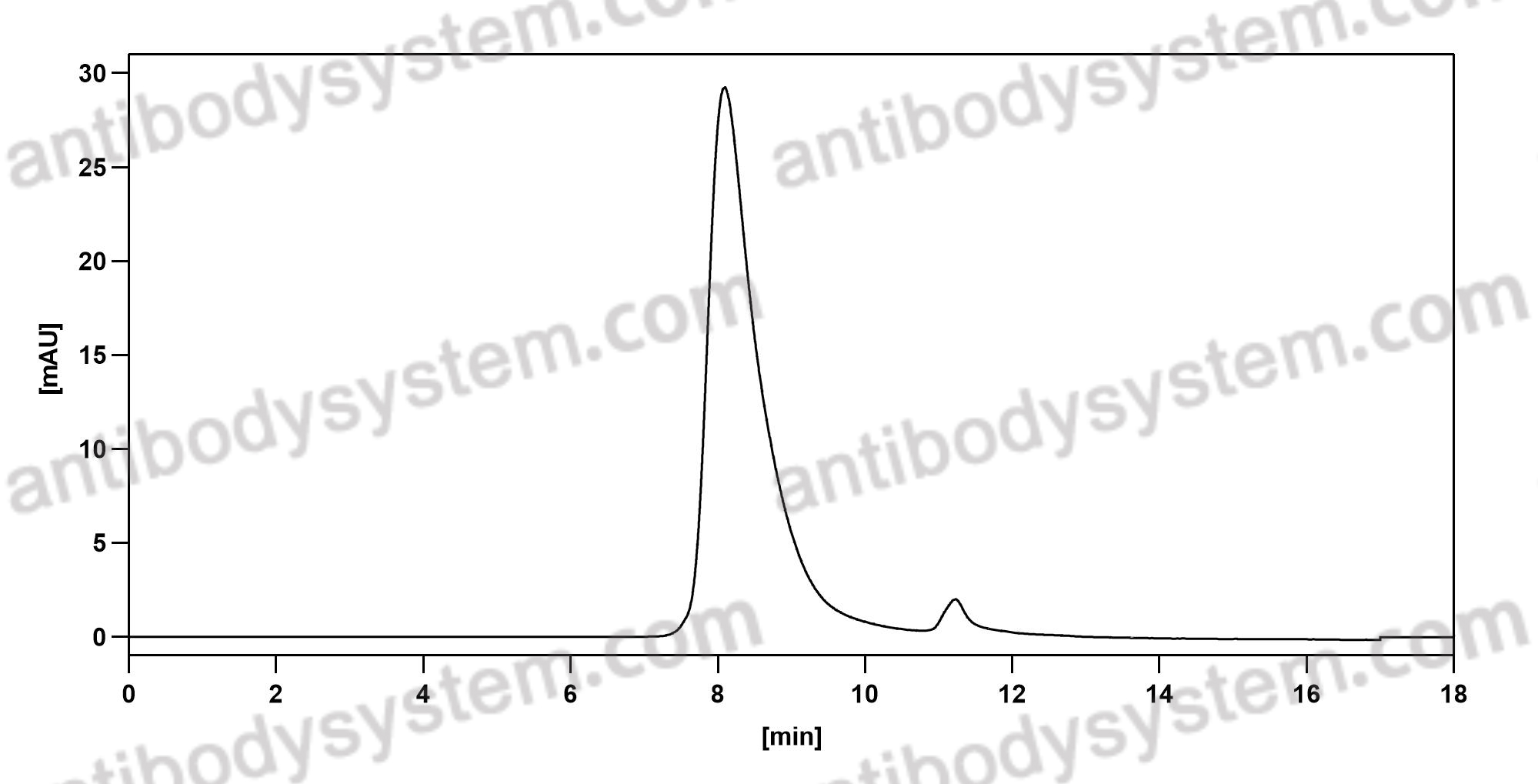
Dinutuximab beta for neuroblastoma, PMID: 33363307
Dinutuximab, PMID: 29999821
Dinutuximab, PMID: 31644006
Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial, PMID: 28549783
The safety of dinutuximab for the treatment of pediatric patients with high-risk neuroblastoma, PMID: 30433831
Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial, PMID: 30442501
Dinutuximab for maintenance therapy in pediatric neuroblastoma, PMID: 28389455
Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children's Oncology Group, PMID: 32343642
Dinutuximab: An Anti-GD2 Monoclonal Antibody for High-Risk Neuroblastoma, PMID: 26917818
Dinutuximab: A Review in High-Risk Neuroblastoma, PMID: 26891967
Dinutuximab: first global approval, PMID: 25940913
Dinutuximab Beta for Treating Neuroblastoma: An Evidence Review Group and Decision Support Unit Perspective of a NICE Single Technology Appraisal, PMID: 30465228
Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: development and place in therapy, PMID: 30613134
Overview and recent advances in the treatment of neuroblastoma, PMID: 28142287
Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1), PMID: 32013055
Local delivery of dinutuximab from lyophilized silk fibroin foams for treatment of an orthotopic neuroblastoma model, PMID: 32096344
Dinutuximab in adult-onset chemotherapy refractory high-risk neuroblastoma, PMID: 32356686
Dinutuximab Synergistically Enhances the Cytotoxicity of Natural Killer Cells to Retinoblastoma Through the Perforin-Granzyme B Pathway, PMID: 32440155
Dinutuximab for the treatment of pediatric patients with high-risk neuroblastoma, PMID: 26934530
Dinutuximab and Panobinostat, PMID: 26912916
GD2 targeting by dinutuximab beta is a promising immunotherapeutic approach against malignant glioma, PMID: 32246395
Dinutuximab for the treatment of pediatric patients with neuroblastoma, PMID: 29238760
Implementation of immunotherapy into the treatment of neuroblastoma - single center experience with the administration of dinutuximab and management of its adverse effects, PMID: 33108882
Nivolumab and dinutuximab beta in two patients with refractory neuroblastoma, PMID: 32414861
Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma, PMID: 20879881
Initiation of immunotherapy with activated natural killer cells and anti-GD2 antibody dinutuximab prior to resection of primary neuroblastoma prolongs survival in mice, PMID: 33428582
Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy, PMID: 32733795
Development of a dinutuximab delivery system using silk foams for GD2 targeted neuroblastoma cell death, PMID: 33252182
Quantification of total dinutuximab concentrations in neuroblastoma patients with liquid chromatography tandem mass spectrometry, PMID: 29938370
PD-L1, inflammation, non-coding RNAs, and neuroblastoma: Immuno-oncology perspective, PMID: 29196189
Tolerance of dinutuximab therapy for treatment of high-risk neuroblastoma in two patients with end-stage renal disease on dialysis, PMID: 33381917
Patients' NK cell stimulation with activated plasmacytoid dendritic cells increases dinutuximab-induced neuroblastoma killing, PMID: 32342128
Activated Natural Killer Cells in Combination with Anti-GD2 Antibody Dinutuximab Improve Survival of Mice after Surgical Resection of Primary Neuroblastoma, PMID: 30232225
The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology, PMID: 33341446
A Case of Anti-NMDA Receptor Encephalitis During Dinutuximab Therapy for Neuroblastoma, PMID: 31651725
Dinutuximab approved for high-risk neuroblastoma, PMID: 25851859
Optimizing care for high-risk neuroblastoma patients treated with dinutuximab: Challenges for the multidisciplinary team, PMID: 32660378
Extensive small bowel pneumatosis and ischemia during dinutuximab therapy for high-risk neuroblastoma, PMID: 31925911
Emerging novel agents for patients with advanced Ewing sarcoma: a report from the Children's Oncology Group (COG) New Agents for Ewing Sarcoma Task Force, PMID: 31031965
Anti-GD2 antibody dinutuximab inhibits triple-negative breast tumor growth by targeting GD2 + breast cancer stem-like cells, PMID: 33722905
Accelerating drug development for neuroblastoma: Summary of the Second Neuroblastoma Drug Development Strategy forum from Innovative Therapies for Children with Cancer and International Society of Paediatric Oncology Europe Neuroblastoma, PMID: 32653773
Impact of IL-2 on Treatment Tolerance in Patients With High-Risk Neuroblastoma Treated With Dinutuximab Beta-Based Immunotherapy, PMID: 33392114
In brief: Dinutuximab (Unituxin) for high-risk neuroblastoma, PMID: 27027692
Dinutuximab: A Novel Immunotherapy in the Treatment of Pediatric Patients With High-Risk Neuroblastoma [Formula: see text], PMID: 27456981
Edoxaban, PMID: 26445907
Treatment and survival of childhood neuroblastoma: Evidence from a population-based study in the United States, PMID: 29039999
The Role of Nursing Professionals in the Management of Patients With High-Risk Neuroblastoma Receiving Dinutuximab Therapy, PMID: 28061552
GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma, PMID: 33303017
G-CSF as a suitable alternative to GM-CSF to boost dinutuximab-mediated neutrophil cytotoxicity in neuroblastoma treatment, PMID: 34049929
A Phase I/IIa Study of Antidisialoganglioside Antibody Dinutuximab in Japanese Patients With Neuroblastoma, PMID: 31815885
Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy., PMID:40507305
Efficacy and Safety of Anti-GD2 Immunotherapy with Dinutuximab Beta in the Treatment of Relapsed/Refractory High-Risk Neuroblastoma., PMID:40459700
Advancements in neuroblastoma treatment: FDA-approved drugs and role of phytochemicals., PMID:40442345
Autophagy inhibition amplifies anti-tumor immunity effect of dinutuximab beta on neuroblastoma via the VEGFR/AKT/mTOR and ROS/NF-κB pathways., PMID:40378433
Ocular Toxicity in GD-2 Antibody Therapy: A Case Study., PMID:40247734
A comprehensive clinico-pathological review of a series of pediatric, adolescents and young adults with high-grade osteosarcoma: from clinics to biomarker discovery., PMID:40198505
A clinical observational study of dinutuximab beta as first-line maintenance treatment for patients with high-risk neuroblastoma in China., PMID:40091019
Management and outcome of children with high-risk neuroblastoma: insights from the Spanish Society of Pediatric Hematology and Oncology (SEHOP) neuroblastoma group on refractory and relapse/progressive disease., PMID:39998749
PHOX2B -associated Congenital Central Hypoventilation Syndrome Revealed Upon Treatment With Dinutuximab-beta., PMID:39961018
Post Transplant Outcomes of High-Risk Neuroblastoma From a Tertiary Care Unit in India., PMID:39912276
Strategies to manage the adverse effects of immunotherapy with dinutuximab beta in neuroblastoma: an Italian experience and literature review., PMID:39907793
Ketamine Infusion as an Adjunct to Opioid Analgesia in Pediatric Patients with High-Risk Neuroblastoma Undergoing Treatment with Dinutuximab: Adverse Effects and Safety in a Non-ICU Setting., PMID:39867536
131I-mIBG therapy in relapsed/refractory neuroblastoma: A weapon from the future past., PMID:39732302
Prospects of anti-GD2 immunotherapy for retinoblastoma., PMID:39620227
Iwilfin (eflornithine) approved by the FDA as the first and only oral maintenance therapy for high-risk neuroblastoma in adult and pediatric patients: Narrative review., PMID:39612452
Case Report of Dinutuximab-induced Atypical Hemolytic Uremic Syndrome., PMID:39530431
GD2 in Breast Cancer: A Potential Biomarker and Therapeutic Target., PMID:39467630
Local Sustained Dinutuximab Delivery and Release From Methacrylated Chondroitin Sulfate., PMID:39359103
Safety and outcome of children, adolescents and young adults participating in phase I/II clinical oncology trials: a 9-year center experience., PMID:39318620
Current Knowledge and Perspectives of Immunotherapies for Neuroblastoma., PMID:39199637
Patterns of recurrence after radiotherapy for high-risk neuroblastoma: Implications for radiation dose and field., PMID:38880415
Targeting both GD2 and B7-H3 using bispecific antibody improves tumor selectivity for GD2-positive tumors., PMID:38853889
Enhancing IgA-mediated neutrophil cytotoxicity against neuroblastoma by CD47 blockade., PMID:38782540
Transition to a mesenchymal state in neuroblastoma may be characterized by a high expression of GD2 and by the acquisition of immune escape from NK cells., PMID:38736882
Autologous hematopoietic stem cell transplantation followed by quadruple immunotherapy with dinutuximab beta, sargramostim, aldesleukin, and spironolactone for relapsed metastatic retinoblastoma., PMID:38679862
Choosing T-cell sources determines CAR-T cell activity in neuroblastoma., PMID:38601159
ECLIM-SEHOP: how to develop a platform to conduct academic trials for childhood cancer., PMID:38600340
TRIB3 silencing promotes the downregulation of Akt pathway and PAX3-FOXO1 in high-risk rhabdomyosarcoma., PMID:38581035
Chemo-immunotherapy with dinutuximab beta in patients with relapsed/progressive high-risk neuroblastoma: does chemotherapy backbone matter?, PMID:38489858
Bilateral tonic pupils secondary to anti-GD2 antibody therapy for neuroblastoma., PMID:38368925
A novel approach to guide GD2-targeted therapy in pediatric tumors by PET and [64Cu]Cu-NOTA-ch14.18/CHO., PMID:38323317
Targeted therapies in retinoblastoma: GD2-directed immunotherapy following autologous stem cell transplantation and evaluation of alternative target B7-H3., PMID:38240863
Persistence of Racial and Ethnic Disparities in Risk and Survival for Patients with Neuroblastoma over Two Decades., PMID:38213818
Yoga as a non-pharmacologic therapy to reduce dinutuximab-induced pain in patients with neuroblastoma., PMID:38192171
Bevacizumab, Irinotecan, or Topotecan Added to Temozolomide for Children With Relapsed and Refractory Neuroblastoma: Results of the ITCC-SIOPEN BEACON-Neuroblastoma Trial., PMID:38190578
Toxicity Spectrum of Anti-GD2 Immunotherapy: A Real-World Study Leveraging the US Food and Drug Administration Adverse Event Reporting System., PMID:38153627
Safety and efficacy of dinutuximab in the treatment of neuroblastoma: A review., PMID:38116487
GM-CSF, G-CSF or no cytokine therapy with anti-GD2 immunotherapy for high-risk neuroblastoma., PMID:38108214
Late Relapse in Neuroblastoma: Case Report and Review of the Literature., PMID:37929737
Eflornithine as Postimmunotherapy Maintenance in High-Risk Neuroblastoma: Externally Controlled, Propensity Score-Matched Survival Outcome Comparisons., PMID:37883734
A Multi-Color Flow Cytometric Assay for Quantifying Dinutuximab Binding to Neuroblastoma Cells in Tumor, Bone Marrow, and Blood., PMID:37834874
Multimodal Therapy with Consolidating Haploidentical Stem Cell Transplantation and Dinutuximab Beta for Patients with High-Risk Neuroblastoma and Central Nervous System Relapse., PMID:37834840
Long-term, continuous infusion of single-agent dinutuximab beta for relapsed/refractory neuroblastoma: an open-label, single-arm, Phase 2 study., PMID:37813959
Exploring dual effects of dinutuximab beta on cell death and proliferation of insulinoma., PMID:37802653
Treatment of High-Risk Neuroblastoma with Dinutuximab and Chemotherapy Administered in all Cycles of Induction., PMID:37760578
Targeting GD2 after allogeneic SCT: effector cell composition defines the optimal use of ch14.18 and the bispecific antibody construct NG-CU (GD2-CD3)., PMID:37742286
Cost-effectiveness analysis of dinutuximab β for the treatment of high-risk neuroblastoma in China., PMID:37715719
Dinutuximab Beta Maintenance Therapy in Patients with High-Risk Neuroblastoma in First-Line and Refractory/Relapsed Settings-Real-World Data., PMID:37629294
The yes-associated protein (YAP) is associated with resistance to anti-GD2 immunotherapy in neuroblastoma through downregulation of ST8SIA1., PMID:37554309