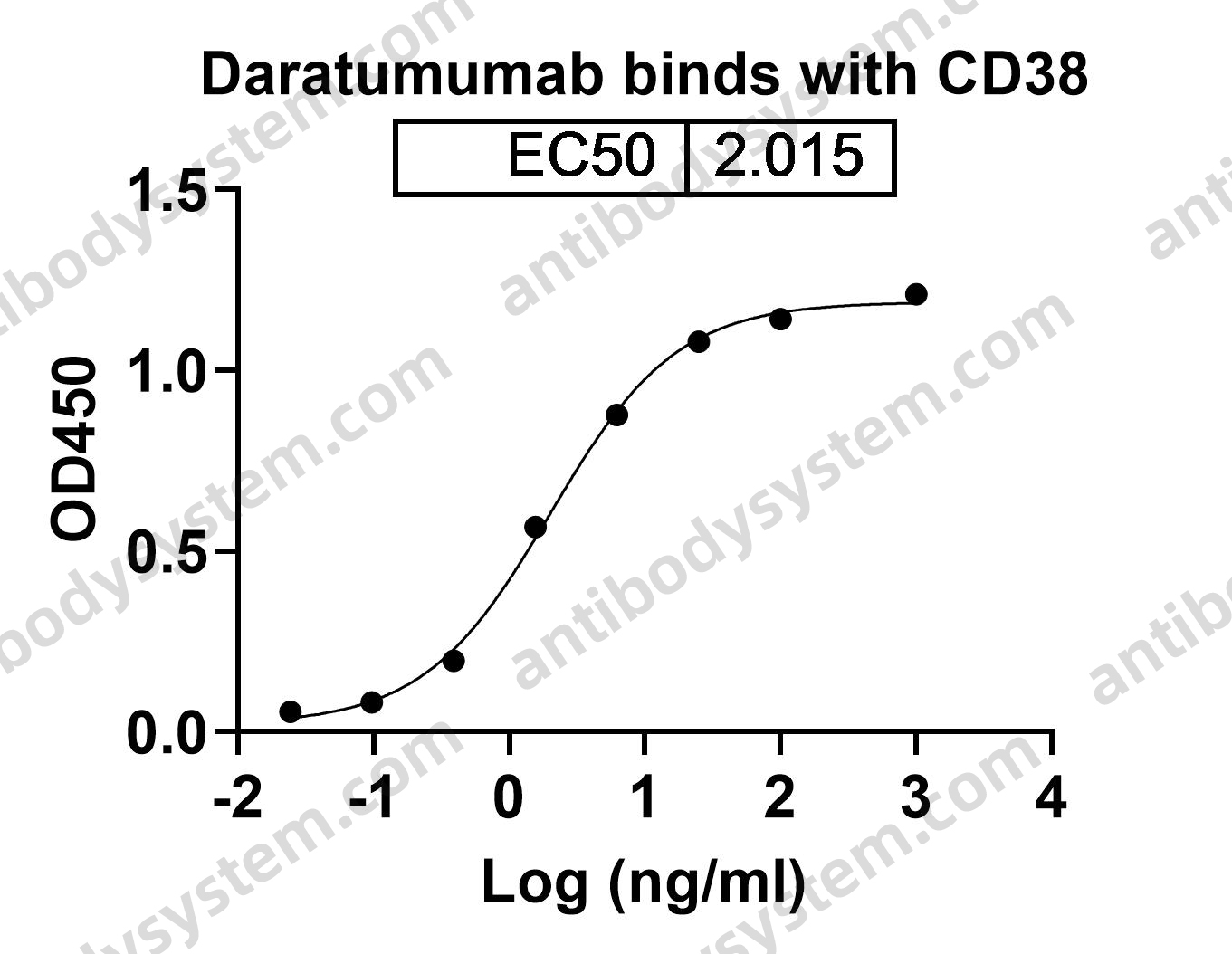
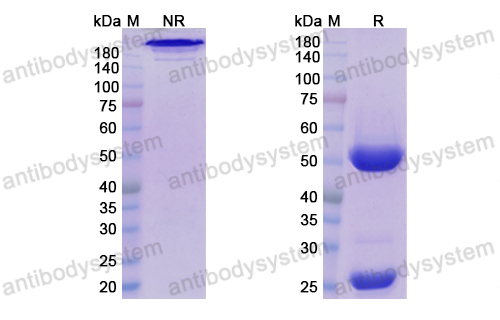
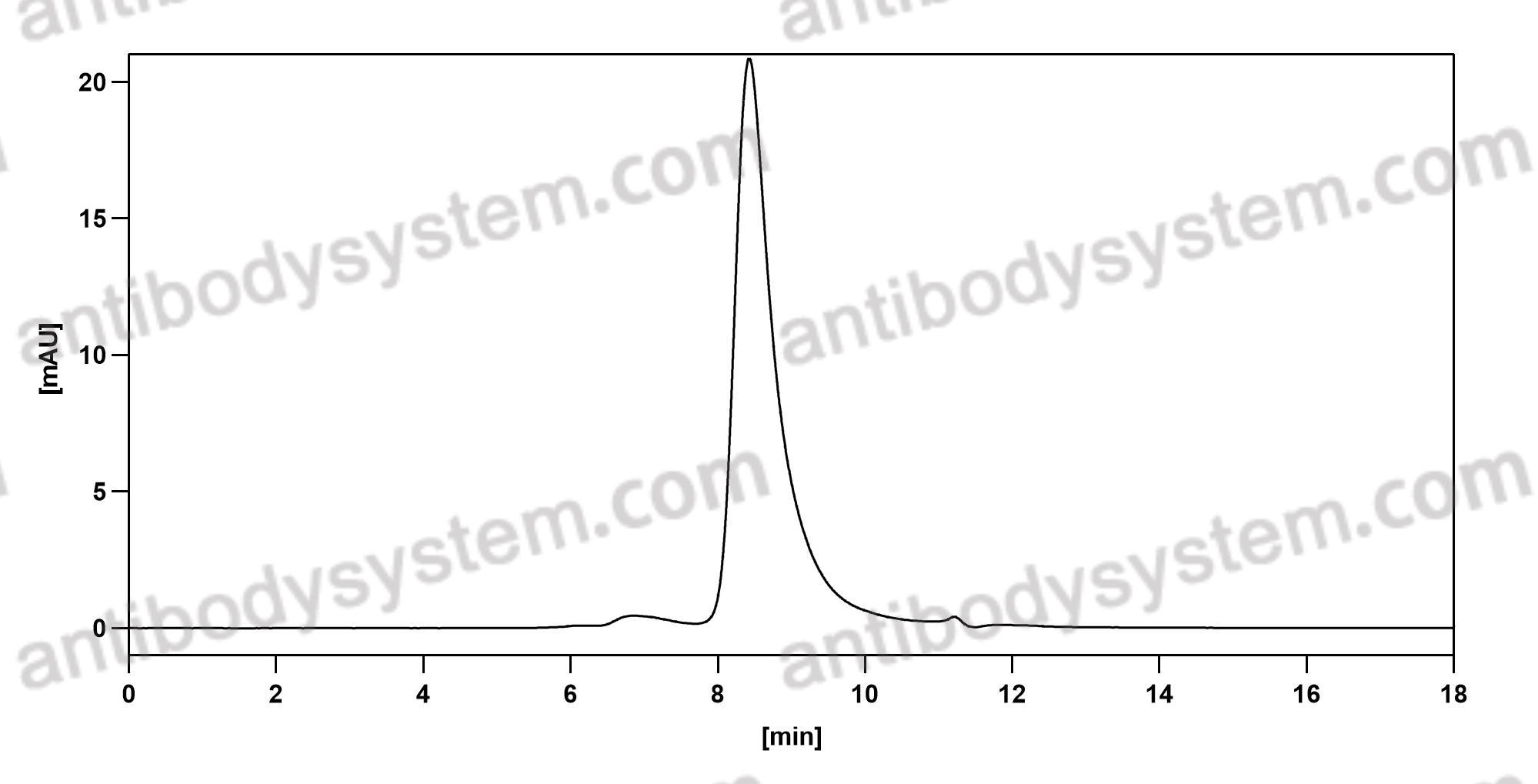
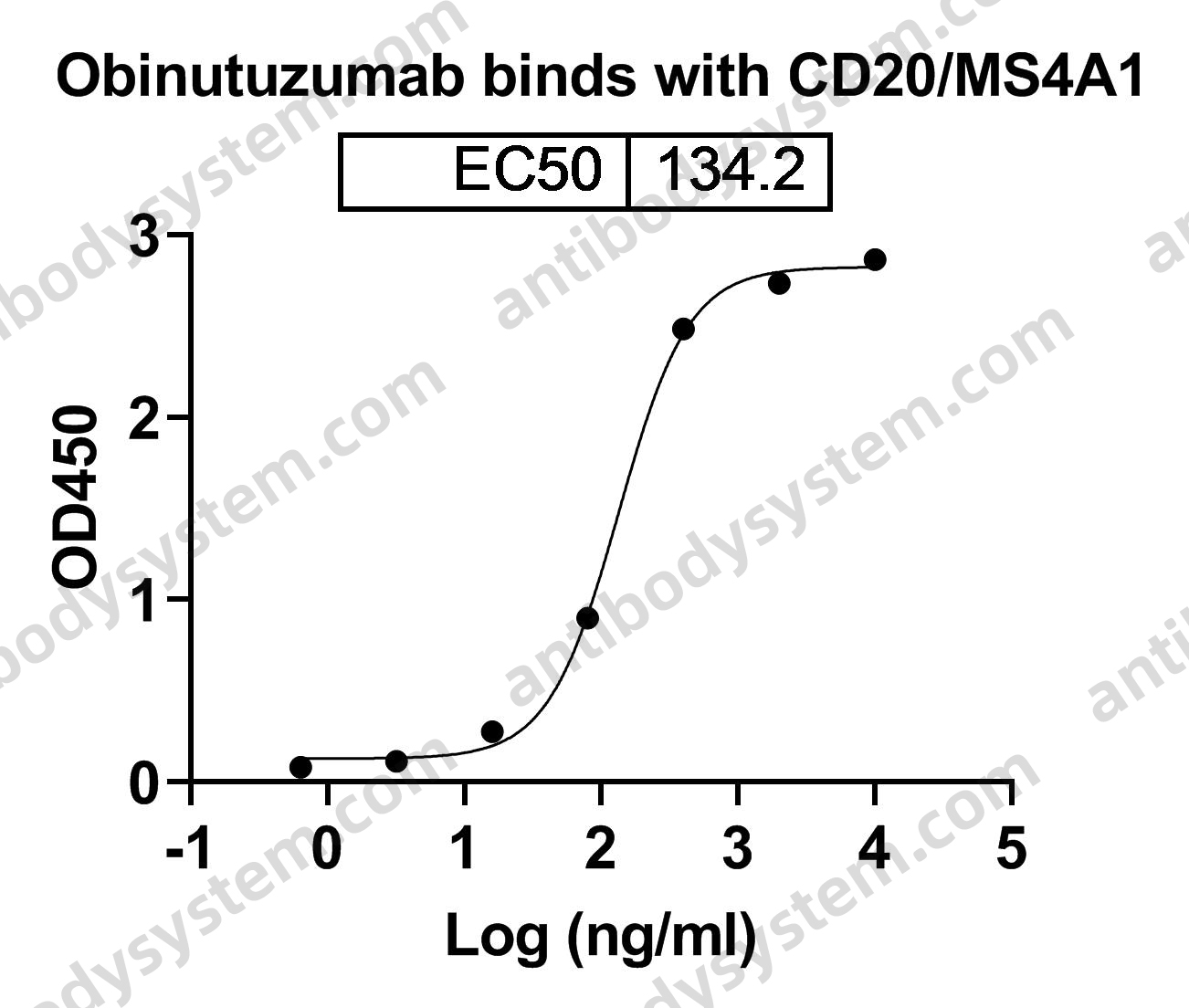
Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma, PMID: 27557302
Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma, PMID: 31141632
Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma, PMID: 27705267
Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA, PMID: 32244252
Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study, PMID: 32682484
Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma, PMID: 29231133
Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma, PMID: 31113777
Daratumumab for the treatment of AL amyloidosis, PMID: 30033840
Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial, PMID: 32213342
Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma, PMID: 28637662
Daratumumab in multiple myeloma, PMID: 30951198
Daratumumab for the treatment of multiple myeloma, PMID: 28434255
Targeting CD38 with Daratumumab in Refractory Systemic Lupus Erythematosus, PMID: 32937047
Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR, PMID: 30237264
Mechanisms of Resistance to Anti-CD38 Daratumumab in Multiple Myeloma, PMID: 31936617
Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial, PMID: 31836199
Daratumumab-based induction therapy for multiple myeloma: A systematic review and meta-analysis, PMID: 33387628
Daratumumab subcutaneous formulation for the treatment of multiple myeloma, PMID: 32750265
Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma, PMID: 31270103
[Daratumumab for multiple myeloma], PMID: 30430991
Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma, PMID: 27222480
Daratumumab: Therapeutic asset, biological trap!, PMID: 29336950
Daratumumab, bortezomib, cyclophosphamide and dexamethasone in newly diagnosed and relapsed multiple myeloma: LYRA study, PMID: 30828799
Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma, PMID: 26308596
Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study, PMID: 32001798
Daratumumab-lenalidomide-dexamethasone vs standard-of-care regimens: Efficacy in transplant-ineligible untreated myeloma, PMID: 32804408
Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX, PMID: 30237262
Daratumumab-based regimens are highly effective and well tolerated in relapsed or refractory multiple myeloma regardless of patient age: subgroup analysis of the phase 3 CASTOR and POLLUX studies, PMID: 31221782
Pomalidomide, dexamethasone, and daratumumab in relapsed refractory multiple myeloma after lenalidomide treatment, PMID: 32376855
Daratumumab for the Treatment of Multiple Myeloma, PMID: 29915586
Daratumumab: A Review in Combination Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma, PMID: 32936436
Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial, PMID: 26778538
Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR, PMID: 32482541
Daratumumab displays in vitro and in vivo anti-tumor activity in models of B-cell non-Hodgkin lymphoma and improves responses to standard chemo-immunotherapy regimens, PMID: 31296574
Amyloidosis: the use of the Daratumumab, PMID: 33331565
CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity, PMID: 32603414
A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis, PMID: 32108228
Daratumumab in high-risk relapsed/refractory multiple myeloma patients: adverse effect of chromosome 1q21 gain/amplification and GEP70 status on outcome, PMID: 31820442
Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia, PMID: 29305553
Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy (PLEIADES): an open-label Phase II study, PMID: 33216361
Daratumumab: A Review in Combination Therapy for Transplant-Ineligible Newly Diagnosed Multiple Myeloma, PMID: 30830601
Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab, PMID: 32457357
An evaluation of subcutaneous daratumumab for the treatment of multiple myeloma, PMID: 32659139
Daratumumab, lenalidomide, and dexamethasone in relapsed/refractory myeloma: a cytogenetic subgroup analysis of POLLUX, PMID: 33149130
Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and SIRIUS trials, PMID: 32470437
Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors, PMID: 21187443
Safety of ninety-minute daratumumab infusion, PMID: 32865161
Daratumumab in transplant regimens for myeloma?, PMID: 32818244
Daratumumab and its use in the treatment of relapsed and/or refractory multiple myeloma, PMID: 30136602
Daratumumab: First Global Approval, PMID: 26729183
Daratumumab (anti-CD38)- and elotuzumab (anti-SLAMF7)-based treatments for refractory POEMS syndrome: a single-center case series., PMID:40528367
A phase 2 study of Daratumumab with Thalidomide and dexamethasone in relapsed and/or Refractory Myeloma (RRMM)., PMID:40527888
CD38-targeted antibody-polymer drug conjugates for enhanced treatment of multiple myeloma., PMID:40516447
Medicare spending and use of subcutaneous biologic formulations with hyaluronidase., PMID:40515473
Biallelic antigen escape is a mechanism of resistance to anti-CD38 antibodies in multiple myeloma., PMID:40493883
[Use of Daratumumab, Carfilzomib and Pomalidomide in Patients with Relapsed Multiple Myeloma: A Current High-Efficacy Treatment]., PMID:40493473
Case Report: BCMA-targeting CAR T-cell therapy induces complete and durable remission in relapsed extramedullary plasmablastic multiple myeloma., PMID:40491928
Daratumumab for Treatment of Acute Antibody-Mediated Rejection in a Pediatric Kidney Transplant Recipient., PMID:40490910
Final Results of a Phase 2 Multi-Arm Study of Magrolimab Combinations in Patients With Relapsed/Refractory Multiple Myeloma., PMID:40485906
Daratumumab for Anti-HLA Antibody Desensitization and Antibody Mediated Rejection in Pediatric Heart Transplant Patients., PMID:40485616
Antibody-mediated ratiometric delivery of FLT3 and CDK4/6 dual inhibitors for targeted treatment of acute myeloid leukemia., PMID:40482924
Comparative Efficacy and Safety of Daratumumab-Integrated Quadruple Therapy Versus Triple Therapy in Transplant-Eligible Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-Analysis., PMID:40473556
Daratumumab for PRCA after HCT: study and practical considerations from the EBMT Transplant Complications Working Party., PMID:40467580
Improved survival with daratumumab-CyBorD compared with CyBorD as frontline therapy for AL amyloidosis., PMID:40453144
Functional multiomics reveals genetic and pharmacologic regulation of surface CD38 in multiple myeloma., PMID:40453054
Interdisciplinary Approach to Diagnostic Challenges: A Case Study of Cardiac Amyloid Light-Chain (AL) Amyloidosis, Multiple Myeloma, and Ductal Carcinoma In Situ., PMID:40452673
The utility of intravenous immunoglobulin infection prophylaxis in patients on anti-CD38 therapies., PMID:40452474
Targeting CD38 in Antibody-Mediated Rejection., PMID:40444214
Social determinants of health and their impact on frontline treatment patterns among Medicare Advantage members with newly diagnosed multiple myeloma., PMID:40442999
Daratumumab and missed sequencing opportunities in transplant-ineligible multiple myeloma: lessons for future trials., PMID:40442311
Clinical Benefit of Early Daratumumab Use in Multiple Myeloma is Undefined., PMID:40439470
Daratumumab in Relapsed or Refractory Pediatric Immune Thrombocytopenia., PMID:40435473
Pure red cell aplasia after ABO incompatible allogeneic stem cell transplantation treated with therapeutic plasma exchange: A case report., PMID:40433827
Ten-year experience on home care for patients with plasma cell disorders: bringing optimal therapy home., PMID:40423883
Real-World Treatment Patterns and Survival Outcomes of Patients with Relapsed/Refractory Multiple Myeloma Treated with a Selinexor-Containing Triplet-Based Regimen., PMID:40422527
Analysis of pharmacotherapeutic approaches for multiple myeloma and correlated renal and pulmonary impairments: a retrospective real-world registry study in the Greater Gulf Region (REPAIR Study)., PMID:40416867
Antibody polymer drug conjugates with increased drug to antibody ratio: CD38-targeting nanomedicines for innovative therapy of relapsed lymphomas., PMID:40409372
Health-related quality of life with daratumumab, bortezomib, melphalan, and prednisone versus bortezomib, melphalan, and prednisone alone in transplant-ineligible patients with newly diagnosed multiple myeloma: analysis of the phase 3 OCTANS study., PMID:40407887
Daratumumab monotherapy as a desensitization strategy prior to cardiac transplantation., PMID:40407767
IGM-7354, an immunocytokine with IL-15 fused to an anti-PD-L1 IgM, induces NK and CD8+ T cell-mediated cytotoxicity of PD-L1 positive tumor cells., PMID:40402523
Successful treatment of refractory cold agglutinin syndrome using fixed duration daratumumab, bortezomib and dexamethasone with a sutimlimab bridge., PMID:40400475
Integrating single-cell and bulk RNA profiles to uncover glutamine metabolism's role in prognosis and immune dynamics in multiple myeloma., PMID:40389878
Optimal daratumumab-based regimen for patients with newly diagnosed and previously untreated multiple myeloma: systematic review and component network meta-analysis., PMID:40380262
Subcutaneous daratumumab monotherapy lowers the risk of progression to active multiple myeloma or death., PMID:40377078
An Update of the Appropriate Sequencing for Salvage Therapies in Multiple Myeloma., PMID:40375908
Addition of Daratumumab to First-Line Therapy of Newly Diagnosed Multiple Myeloma in Patients Eligible for Autologous Stem Cell Transplantation: A Single-Center Real-Life Experience., PMID:40375903
Trend of treatment strategy for amyloid light-chain amyloidosis: a-single center experience., PMID:40372551
Impact of the Addition of Daratumumab to the Frontline Treatment of Patients with Immunoglobulin Light-Chain Amyloidosis: A Single-Centre Experience., PMID:40361367
Real-World Treatment Outcomes of Different Sequencing Options with Daratumumab, Lenalidomide, and Dexamethasone in Patients with Transplant-Ineligible Multiple Myeloma in Japan., PMID:40361315
Practice-changing updates on multiple myeloma: highlights from the 2024 ASH annual meeting., PMID:40361170
Cytomegalovirus infection after autologous transplantation in patients with multiple myeloma exposed to daratumumab., PMID:40360761
Daratumumab for newly diagnosed multiple myeloma: Pooled analysis of patients aged ≥65 years from GRIFFIN and PERSEUS., PMID:40360369
Using bispecific antibodies in a patient with peritoneal dialysis for relapsed multiple myeloma., PMID:40356496
Relapsed/refractory multiple myeloma: standard of care management of patients in the Gulf region., PMID:40352314
EXCALIBER-RRMM: a phase III trial of iberdomide, daratumumab, and dexamethasone in relapsed/refractory multiple myeloma., PMID:40346992
Stem Cell Mobilization Yields with Daratumumab (Dara) and Lenalidomide (Len)-Containing Quadruplet Induction Therapy in Patients with Newly Diagnosed Multiple Myeloma (NDMM): A Real-World Experience at 2 Institutes., PMID:40340128
Real-world analysis of treatment patterns, effectiveness, and safety of daratumumab-based regimens in Chinese patients with newly diagnosed or relapsed/refractory multiple myeloma., PMID:40335949
Targeting CD38 with Daratumumab for Platelet Transfusion Refractoriness in Aplastic Anemia., PMID:40331918
Daratumumab, Venetoclax, and Azacitidine in Combination with HAA Regimen as Consolidation Chemotherapy for T-cell Acute Lymphoblastic Leukemia with High CD38 Expression., PMID:40326525
[The Clinical Characteristics and Prognosis of Patients with Light-Chain Amyloidosis: A Retrospective Analysis]., PMID:40326140