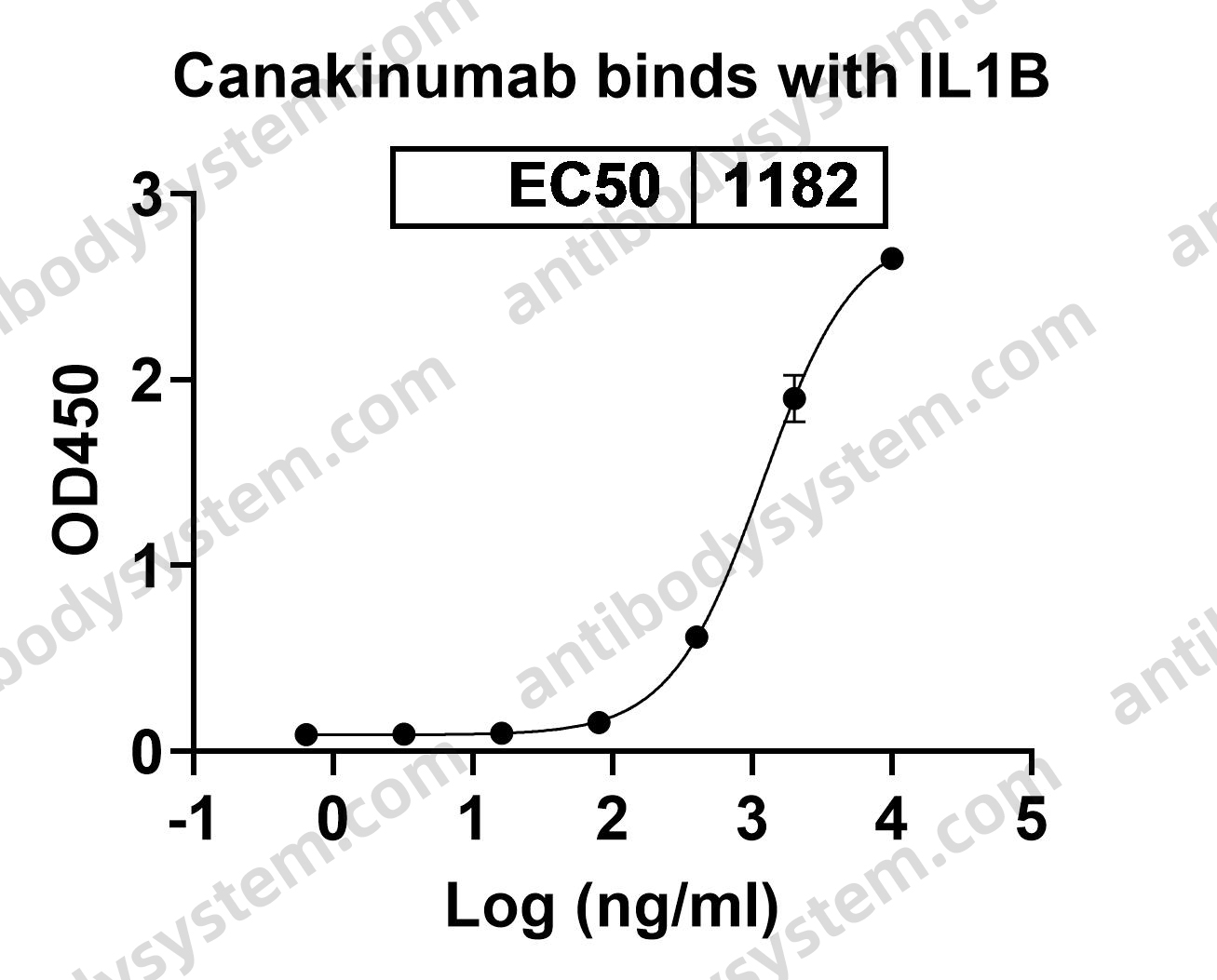
Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease, PMID: 28845751
Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes, PMID: 29768139
Canakinumab as treatment for COVID-19-related pneumonia: A prospective case-control study, PMID: 33385581
Canakinumab, PMID: 31643219
Blockage of interleukin-1β with canakinumab in patients with Covid-19, PMID: 33311551
Canakinumab for the treatment of adult-onset Still's disease, PMID: 31957508
Canakinumab, PMID: 20065636
Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis, PMID: 23252526
Canakinumab, PMID: 29999640
Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes, PMID: 29544870
Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial, PMID: 28855077
Canakinumab for Treatment of Adult-Onset Still's Disease to Achieve Reduction of Arthritic Manifestation (CONSIDER): phase II, randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial, PMID: 32404342
Tapering Canakinumab Monotherapy in Patients With Systemic Juvenile Idiopathic Arthritis in Clinical Remission: Results From a Phase IIIb/IV Open-Label, Randomized Study, PMID: 32783351
Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure, PMID: 30586730
Canakinumab for the treatment of hyperimmunoglobulin D syndrome, PMID: 30652926
Canakinumab in gout, PMID: 22784099
Canakinumab: Promises and Future in Cardiometabolic Diseases and Malignancy, PMID: 30832770
Dosage Considerations for Canakinumab in Children With Periodic Fever Syndromes, PMID: 30447083
Expedited Desensitization to Canakinumab, PMID: 32612876
Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS), PMID: 30165610
Efficacy and safety of canakinumab treatment in schnitzler syndrome: A systematic literature review, PMID: 32502728
Canakinumab in colchicine resistant familial Mediterranean fever and other pediatric rheumatic diseases, PMID: 32419407
COVID-19 revisiting inflammatory pathways of arthritis, PMID: 32561873
Canakinumab for secondary prevention of atherosclerotic disease, PMID: 29265905
Inhibition of IL1β by Canakinumab May Be Effective against Diverse Molecular Subtypes of Lung Cancer: An Exploratory Analysis of the CANTOS Trial, PMID: 33023946
Effect of canakinumab on clinical and biochemical parameters in acute gouty arthritis: a meta-analysis, PMID: 32918702
A review of canakinumab and its therapeutic potential for non-small cell lung cancer, PMID: 31503012
Canakinumab for Atherosclerotic Disease, PMID: 29322757
Canakinumab for Atherosclerotic Disease, PMID: 29322754
Canakinumab for Atherosclerotic Disease, PMID: 29322758
Canakinumab for Atherosclerotic Disease, PMID: 29320648
Canakinumab for Atherosclerotic Disease, PMID: 29322756
Canakinumab for Atherosclerotic Disease, PMID: 29322755
The Challenges of Approaching and Managing Gout, PMID: 30447743
Cost-effectiveness of Canakinumab for Prevention of Recurrent Cardiovascular Events, PMID: 30649147
Canakinumab for the treatment of familial Mediterranean fever, PMID: 28362189
Canakinumab: in patients with cryopyrin-associated periodic syndromes, PMID: 22168385
Canakinumab for the treatment of active systemic juvenile idiopathic arthritis, PMID: 27367267
NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis, PMID: 29880500
Canakinumab and Lung Cancer: Intriguing, but Is It Real?, PMID: 29666299
Canakinumab for the treatment of TNF-receptor associated periodic syndrome, PMID: 28454496
Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial, PMID: 29146124
Validating canakinumab therapy for autoinflammation, PMID: 29844383
Familial Mediterranean Fever, PMID: 30686512
Macrophage activation syndrome in the era of biologic therapy, PMID: 27009539
Long-term efficacy of canakinumab in the treatment of Schnitzler syndrome, PMID: 31940469
Canakinumab Lacks Efficacy in Treating Adult Patients with Moderate to Severe Chronic Spontaneous Urticaria in a Phase II Randomized Double-Blind Placebo-Controlled Single-Center Study, PMID: 32827729
Canakinumab in Children with Familial Mediterranean Fever: A Single-Center, Retrospective Analysis, PMID: 31463794
Interference of canakinumab with commercial IL-1β ELISAs, PMID: 31096037
Canakinumab: a review of its use in the management of systemic juvenile idiopathic arthritis, PMID: 25822149
Blockade of IL-1 family cytokines in the treatment of rheumatoid arthritis., PMID:40520203
Interleukin-1β in circulating mononuclear cells predicts steatotic liver disease improvement after weight loss in subjects with obesity and prediabetes or type 2 diabetes., PMID:40514652
Unmasking the Masquerade: A Case Report of Adult-Onset Still's Disease., PMID:40486417
Is It Time to Revisit the Role of Interleukin-1 Inhibitors in Osteoarthritis?, PMID:40458527
The first nationwide epidemiological survey of chronic recurrent multifocal osteomyelitis in Japan., PMID:40445191
Canakinumab treatment patterns in sJIA, FMF, TRAPS, and MKD/HIDS: real-world insights from a Belgian non-interventional study., PMID:40442768
Canakinumab for the treatment of postprandial hypoglycaemia: study protocol for a randomised, placebo-controlled, parallel-group, double-blind, multicentric, superiority trial-the CanpHy study., PMID:40425249
Impact of Interleukin-1 Blockade on the Development of Macrophage Activation Syndrome in Still's Disease: Incidence and Diagnostic Validity of the EULAR/ACR/PRINTO 2016 MAS Classification Criteria., PMID:40420428
Pregnancy outcomes after maternal and paternal anti-IL-1 treatment exposure in cryopyrin associated periodic syndromes (CAPS)., PMID:40411758
Diagnosis, management, and monitoring of interleukin-1 mediated diseases in Central and Eastern Europe: real-world data., PMID:40405263
Pilot study of canakinumab (Ilaris) in steroid naïve children with Duchenne muscular dystrophy demonstrates safety and exploratory changes in potential serum protein response biomarkers., PMID:40396427
Neutrophils predominate as IL1B-expressing cells in Schnitzler syndrome: Insights from the SCan study to evaluate the efficacy and safety of canakinumab in Japanese patients., PMID:40393905
Pulmonary arterial hypertension in adults with Still's disease: another pulmonary manifestation associated with HLA-DRB1*15., PMID:40379524
Targeting the NLRP3 inflammasome for inflammatory disease therapy., PMID:40374417
Post-marketing safety of anakinra and canakinumab: a real-world pharmacovigilance study based on FDA adverse event reporting system., PMID:40371323
Identification of Hub Genes and Pathways in Preinfusion Chimeric Antigen Receptor (CAR) T-cell Products Associated With Cytokine Release Syndrome., PMID:40370902
Endogenous lipoid pneumonia in adult autoinflammatory disease., PMID:40368851
Targeting IL-6 in antibody-mediated kidney transplant rejection., PMID:40357502
Current anti-inflammatory strategies for treatment of heart failure: From innate to adaptive immunity., PMID:40348101
Contemporary management paradigms and emerging therapeutics for myelodysplastic syndromes/neoplasms., PMID:40302714
Canakinumab for the management of pyoderma gangrenosum: a case series of two patients and literature review., PMID:40302177
Buzzing Away Pain: Efficacy of Buzzy® in Reducing Pain During Canakinumab Treatment for Familial Mediterranean Fever., PMID:40291186
Shared Mechanisms in Cancer and Cardiovascular Disease: S100A8/9 and the NLRP3 Inflammasome: JACC: CardioOncology State-of-the-Art Review., PMID:40260700
Long-term efficacy and safety of colchicine and anti-IL-1 blockers in FMF: results from the Eurofever multicenter observational study., PMID:40252570
Tumor Necrosis Factor Receptor-Associated Periodic Syndrome: Genetics, Autoinflammation, and Recurrent Pericarditis., PMID:40250904
Seeking and treating inflammation in ischaemic heart disease: are we ready?, PMID:40248283
Successful Treatment of PAPASH Syndrome With Concomitant FMF Using IL-1 Blockade: A Case Report., PMID:40243006
Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis., PMID:40215252
The Efficacy and Safety of Hepatitis A Vaccine in Children and Young Adults With an Autoinflammatory Diseases on Canakinumab and Tocilizumab Treatments: A Prospective Observational Controlled Study., PMID:40172610
First-line biological versus conventional synthetic disease-modifying antirheumatic drug therapy in adult-onset Still's disease: a multicentre, retrospective, propensity weighted cohort study., PMID:40164168
Current therapeutic options for adult patients with urticarial vasculitis: A scoping review., PMID:40157507
Use of Biologic Therapy in AA Amyloidosis Patients Undergoing Dialysis-A Systematic Literature Review., PMID:40152016
Treatment of uveitis in Blau syndrome: A systematic review and meta-analysis., PMID:40147219
The Impact of the Tumor Microenvironment on the Effect of IL-1β Blockade in NSCLC: Biomarker Analyses from CANOPY-1 and CANOPY-N Trials., PMID:40116353
Two Novel Variants in the LRR Domain of NLRP3 Causing Leukoencephalopathy: A Case Report., PMID:40116107
Use of Anti-interleukin-1 Agents in Kidney Transplant Recipients with Familial Mediterranean Fever and Amyloidosis: What have been learned so far?, PMID:40092658
The Golden Card of Interleukin-1 Blockers in Systemic Inflammasomopathies of Childhood., PMID:40076498
Deficiency of interleukin-1 receptor antagonist: A systematic review., PMID:40060136
The effect of IL-1β inhibitor canakinumab (Ilaris®) on IL-6 production in human skeletal muscle cells., PMID:40048444
Efficacy and safety profile of biotechnological agents and Janus kinase inhibitors in VEXAS syndrome: data from the international AIDA Network VEXAS registry., PMID:40046741
Successful control of recurrent MAS by canakinumab in a Sjogren syndrome patient with homozygous MEFV P369S variants, and review of literatures., PMID:40045586
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
Canakinumab Treatment in Familial Mediterranean Fever Patients: With/Without Colchicine., PMID:40040547
Elevated C-reactive protein and cardiovascular risk., PMID:40014057
Balancing Effective Treatments With Potential Threats: The Impact of Biologic Agent Use on Tuberculosis Development in Children With Chronic Inflammatory Disorders., PMID:39989306
Discovery and Phase 1 study of a novel monoclonal antibody against human IL-1β for the treatment of IL-1β-mediated diseases., PMID:39964852
Blood molecular subtypes to guide precision treatment strategies in systemic juvenile idiopathic arthritis., PMID:39923112
Are participants in gout medication registration clinical trials representative of people with gout in the general population?, PMID:39919487
Associations of Serum Urate and Cardiovascular Events in a Clinical Trial of Interleukin-1β Blockade., PMID:39862678
Post-Marketing Pharmacovigilance of Canakinumab from the FDA Adverse Event Reporting System (FAERS)., PMID:39861175