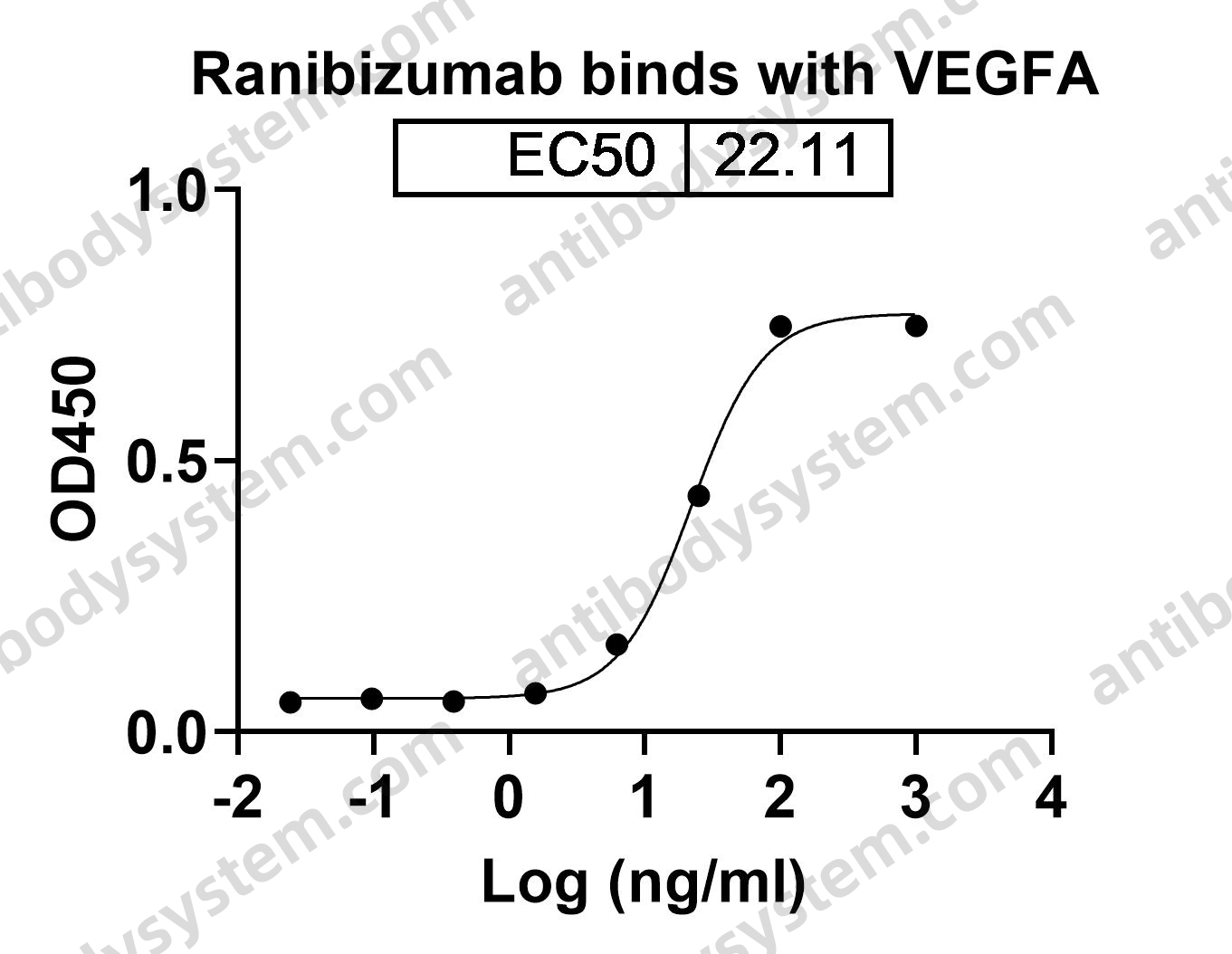
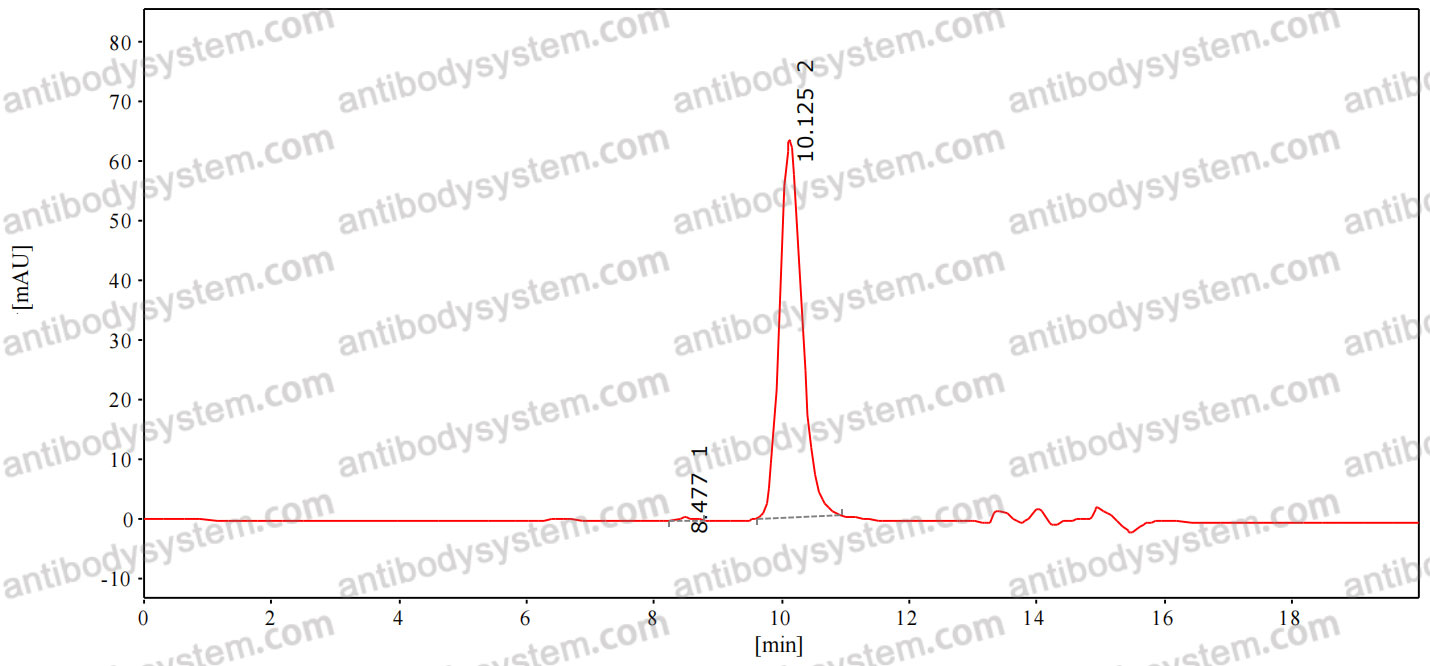
Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial, PMID: 30043039
Ranibizumab for myopic choroidal neovascularization, PMID: 33003962
Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T, PMID: 28837425
Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial, PMID: 31522845
Ranibizumab: A Review in Retinopathy of Prematurity, PMID: 33447937
The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial, PMID: 30946888
Comparative efficacy of bevacizumab, ranibizumab, and aflibercept for treatment of macular edema secondary to retinal vein occlusion: a systematic review and network meta-analysis, PMID: 30071180
Effectiveness and safety of ranibizumab 0.5 mg in treatment-naïve patients with diabetic macular edema: Results from the real-world global LUMINOUS study, PMID: 32492069
The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration-A cost-effectiveness analysis from a societal perspective, PMID: 29772018
Visual Field Changes Over 5 Years in Patients Treated With Panretinal Photocoagulation or Ranibizumab for Proliferative Diabetic Retinopathy, PMID: 31999300
Efficacy and Treatment Burden of Intravitreal Aflibercept Versus Intravitreal Ranibizumab Treat-and-Extend Regimens at 2 Years: Network Meta-Analysis Incorporating Individual Patient Data Meta-Regression and Matching-Adjusted Indirect Comparison, PMID: 32222903
Clinical effectiveness of ranibizumab and conbercept for neovascular age-related macular degeneration: a meta-analysis, PMID: 30464394
Ranibizumab for age-related macular degeneration, PMID: 22309606
Ranibizumab and digital ischemia, PMID: 29425544
Assessing the role of ranibizumab in improving the outcome of glaucoma filtering surgery and neovascular glaucoma, PMID: 29781319
Ranibizumab and aflibercept for the treatment of wet age-related macular degeneration, PMID: 26076760
Switching between ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration, PMID: 29476754
Efficacy and Safety of a Proposed Ranibizumab Biosimilar Product vs a Reference Ranibizumab Product for Patients With Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial, PMID: 33211076
Ranibizumab Population Pharmacokinetics and Free VEGF Pharmacodynamics in Preterm Infants With Retinopathy of Prematurity in the RAINBOW Trial, PMID: 32855889
[Ranibizumab and aflibercept for diabetic macular edema-retrospective study with real-life data after 12 months], PMID: 31705192
Intravitreal ranibizumab and dexamethasone implant injections as primary treatment of diabetic macular edema: simultaneously double protocol, PMID: 32398852
Real-World Effectiveness and Real-World Cost-Effectiveness of Intravitreal Aflibercept and Intravitreal Ranibizumab in Neovascular Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Real-World Studies, PMID: 31728825
Intraocular Pharmacokinetics of 10-fold Intravitreal Ranibizumab Injection Dose in Rabbits, PMID: 32818095
Ranibizumab plus fufang xueshuantong capsule versus ranibizumab alone for exudative age-related macular degeneration, PMID: 32962487
Ranibizumab for the treatment of wet AMD: a summary of real-world studies, PMID: 26634711
Intravitreal Ranibizumab Injection for the Treatment of Retinopathy of Prematurity, PMID: 30995663
Ranibizumab treatment patterns in prior ranibizumab-treated neovascular age-related macular degeneration patients: Real-world outcomes from the LUMINOUS study, PMID: 33378369
Therapeutic Monoclonal Antibodies and Fragments: Ranibizumab, PMID: 26501149
Photodynamic therapy in combination with ranibizumab versus ranibizumab monotherapy for wet age-related macular degeneration: A systematic review and meta-analysis, PMID: 29753123
Renal Biomarkers for Treatment Effect of Ranibizumab for Diabetic Macular Edema, PMID: 32908935
Ranibizumab in the Treatment of Diabetic Macular Edema: A Review of the Current Status, Unmet Needs, and Emerging Challenges, PMID: 28484955
Real-world effectiveness and safety of ranibizumab for the treatment of myopic choroidal neovascularization: Results from the LUMINOUS study, PMID: 31961888
Intravitreal Ranibizumab for the Treatment of Visual Impairment Due to Choroidal Neovascularization Associated with Rare Diseases: Cost-Effectiveness in the UK, PMID: 30726549
Intravitreal ranibizumab reduced ocular blood flow and aqueous cytokine levels and improved retinal morphology in patients with diabetic macular edema, PMID: 33303870
Post-Ranibizumab injection endophthalmitis in aggressive posterior retinopathy of prematurity, PMID: 31124535
Ranibizumab for Macular Edema Secondary to Central and Branch Retinal Vein Occlusion in Patients Younger Than 50 Years of Age, PMID: 32685489
Aflibercept for neovascular age-related macular degeneration, PMID: 26857947
Ranibizumab port delivery system (RPDS): realising long awaited dream of prolonged VEGF suppression, PMID: 31165769
Switching from ranibizumab and aflibercept to bevacizumab therapy in neovascular age-related macular degeneration patients, PMID: 32375502
Cost-Minimization Analysis of Ranibizumab Versus Aflibercept for Treating Saudi Patients With Visual Impairment Owing to Age-Related Macular Degeneration or Diabetic Macular Edema, PMID: 32247191
Biomechanical properties of the cornea following intravitreal ranibizumab injection, PMID: 33165643
Therapeutic effects of ranibizumab in patients with polypoidal choroidal vasculopathy, PMID: 31324161
Cardiovascular risk in patients receiving ranibizumab for exudative age-related macular degeneration: a nationwide self-controlled case-series study, PMID: 32522792
Outcomes and costs of Ranibizumab and Aflibercept treatment in a health-service research context, PMID: 29486762
Evaluation of ranibizumab and aflibercept for the treatment of diabetic macular edema in daily clinical practice, PMID: 31644594
Ranibizumab in macular oedema secondary to branch retinal vein occlusion - 24 months of treatment, PMID: 32397721
Use of Bevacizumab and Ranibizumab for Wet Age-Related Macular Degeneration: Influence of CATT Results and Introduction of Aflibercept, PMID: 31100217
Ranibizumab for the treatment of choroidal neovascularization due to cause other than age related macular degeneration, PMID: 31779462
Real-world outcomes with ranibizumab in branch retinal vein occlusion: The prospective, global, LUMINOUS study, PMID: 32555630
Effect of intravitreal ranibizumab pretreatment on vitrectomy in young patients with proliferative diabetic retinopathy, PMID: 32005066
Comment on: 'Comparing safety and efficacy of Bevacizumab, Ranibizumab and Ranibizumab biosimilar in Retinopathy of prematurity'., PMID:40527930
Unusual presentation of retinitis pigmentosa with vasoproliferative tumour., PMID:40527540
Clinical Trials of Aflibercept Biosimilars: A Review., PMID:40526023
Interventions for central serous chorioretinopathy: a network meta-analysis., PMID:40522203
Real-World 5-Year Outcomes of Age-Related Macular Degeneration with Bevacizumab as First-Line Anti-VEGF., PMID:40517351
Six-year findings of polypoidal choroidal vasculopathy in the EVEREST II study: Japanese subgroup analysis., PMID:40504425
Efficacy of switching from existing anti-vascular endothelial growth factor drugs to ranibizumab biosimilar in neovascular age-related macular degeneration., PMID:40500585
Usage of brolucizumab as treatment for wet age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV): A narrative review., PMID:40489855
Effect of ranibizumab on diabetic retinopathy via the vascular endothelial growth factor/STAT3/glial fibrillary acidic protein pathway., PMID:40487605
Response to 'Comment on: 'Comparing safety and efficacy of Bevacizumab, Ranibizumab and Ranibizumab biosimilar in Retinopathy of prematurity"., PMID:40483306
Anti-VEGFs for Diabetic Macular Oedema: Analysis of Efficacy, Safety, and Cost of More Durable Therapies from a Dutch Societal Perspective., PMID:40481910
Intravitreal anti-vascular endothelial growth factor injections and risks of stroke in patients with neovascular age-related macular degeneration-A registry-based cohort study., PMID:40481786
Modeling the Ocular Pharmacokinetics and Pharmacodynamics of Ranibizumab for Improved Understanding and Data Collection Strategies in Ocular Diseases., PMID:40478563
EFFICACY AND PROGNOSIS OF ANTI-VEGF AGENTS COMBINED WITH PANRETINAL PHOTOCOAGULATION IN DIABETIC RETINOPATHY: A CLINICAL OBSERVATIONAL STUDY., PMID:40466685
Infographic: intravitreal therapy for uveitic macular edema-ranibizumab versus methotrexate versus the dexamethasone implant: the MERIT trial results., PMID:40457097
Association between intravitreal anti-vascular endothelial growth factor agents and hypertension: a meta-analysis., PMID:40456283
Anti-VEGF Agents Clearance Through the Aqueous Outflow Pathway in a Rat Model., PMID:40455045
Several Common Genetic Variations Associate With Functional or Anatomic Effects of Anti-VEGF Treatment in Conditions With Macular Edema., PMID:40455044
Targeting VEGF With 99mTc-Labeled Ranibizumab for Noninvasive Diagnosis of Non-Small Cell Lung Cancer., PMID:40444333
Managing Choroidal Neovascularization in Pseudoxanthoma Elasticum: Outcomes of Vitrectomy and Intravitreal Ranibizumab/Aflibercept Therapy-A Case Report., PMID:40438152
Oral lamivudine in diabetic macular edema: A randomized, double-blind, placebo-controlled clinical trial., PMID:40436012
Area Under the Curve Analysis in a Real-World Cohort of Finnish Patients Treated for Neovascular Age-Related Macular Degeneration., PMID:40423616
Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration., PMID:40423060
Effectiveness and safety of anti-vascular endothelial growth factor therapies for macular edema in retinal vein occlusion: A systematic review and network meta-analysis of randomized controlled trials., PMID:40419166
Subretinal tissue plasminogen activator in polypoidal choroidal vasculopathy-related submacular haemorrhage and exudative retinal detachment., PMID:40409770
Efficacy and safety of prophylactic simultaneous intravitreal moxifloxacin injection with standard intravitreal anti-VEGF injection procedure in cases of cystoid macular edema and macular neovascularization., PMID:40405143
Comparison of three loading doses of bevacizumab and ranibizumab for macular edema due to branch retinal vein occlusion., PMID:40405136
[Age-related macular degeneration - Part 2: therapeutic options for AMD]., PMID:40389215
A Study of Visual Outcomes and Spectral Domain Optical Coherence Tomography (SD-OCT) Biomarker Changes in Patients Treated With Ranibizumab for Diabetic Macular Edema in a Tertiary Hospital., PMID:40385737
Effect of simultaneous intravitreal ranibizumab and extended-release dexamethasone injection on patients with naïve versus refractory retinal vein occlusion macular edema: a prospective, multicenter, and interventional open-label study., PMID:40385115
Socioeconomic Disparities in Intravitreal Injection Use and Anti-VEGF Agent Selection: Aflibercept/Ranibizumab Versus Bevacizumab., PMID:40383690
Combining ranibizumab with calcium dobesilate to reduce injection frequency in diabetic macular edema treatment., PMID:40381058
Therapeutic outcomes of ranibizumab for zone ii stage 2 retinopathy of prematurity with plus disease., PMID:40377801
Recurrence of Macular Edema in Branch Retinal Vein Occlusion-A Comparison of Aflibercept and Ranibizumab in a Randomized Trial., PMID:40373873
Sustainable Practices in Anti-VEGF Therapy: A 15-Year Bibliometric Analysis of Ranibizumab for Age-Related Macular Degeneration., PMID:40365539
Planned vs. Performed Treatment Regimens in Diabetic Macular Edema: Real-World Evidence from the PACIFIC Study., PMID:40364151
Dynamic Changes of Aqueous Matrix Metalloproteinases and Imaging Correlation in Retinal Vein Occlusion Following Ranibizumab Therapy., PMID:40359464
Silicone oil microdroplets from syringes with intravitreal anti-vascular endothelial growth factor and complement inhibitor injections., PMID:40359347
OCTA as a reliable prognostic tool for active NVD treated with Panretinal Photocoagulation and/or ranibizumab., PMID:40359330
[Biosimilars of ranibizumab in retinal diseases: new possibilities in ophthalmology]., PMID:40353548
Long-term outcomes of the observe-and-plan regimen in treating neovascular age-related macular degeneration: a retrospective real-life analysis., PMID:40348918
Anti-VEGF drugs compared with laser photocoagulation for the treatment of diabetic retinopathy: a systematic review and economic analysis., PMID:40347224
PDVR stage A management: Insights from combined PRP and anti-VEGF treatment outcomes., PMID:40340516
Improved functional and morphological outcomes with faricimab in nAMD eyes with poor response to prior intravitreal anti-VEGF therapy., PMID:40338383
Contributing factors to short-term intraocular pressure elevation following intravitreal anti-VEGF injections., PMID:40335957
Endophthalmitis following intravitreal injections at a tertiary center: real-life data on incidence, risk factors, management strategies, and visual outcomes., PMID:40335933
Visual fields after anti-vascular endothelial growth factor therapy for retinopathy of prematurity., PMID:40333931
Efficacy of ranibizumab with laser in the treatment of diabetic retinopathy compare with laser monotherapy: A systematic review and meta-analysis., PMID:40331557
Uncommon Therapeutic Approaches for Patients with Recurrent Central Serous Chorioretinopathy: case report and literature review., PMID:40330964