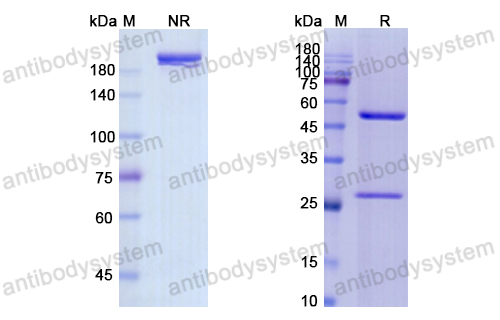
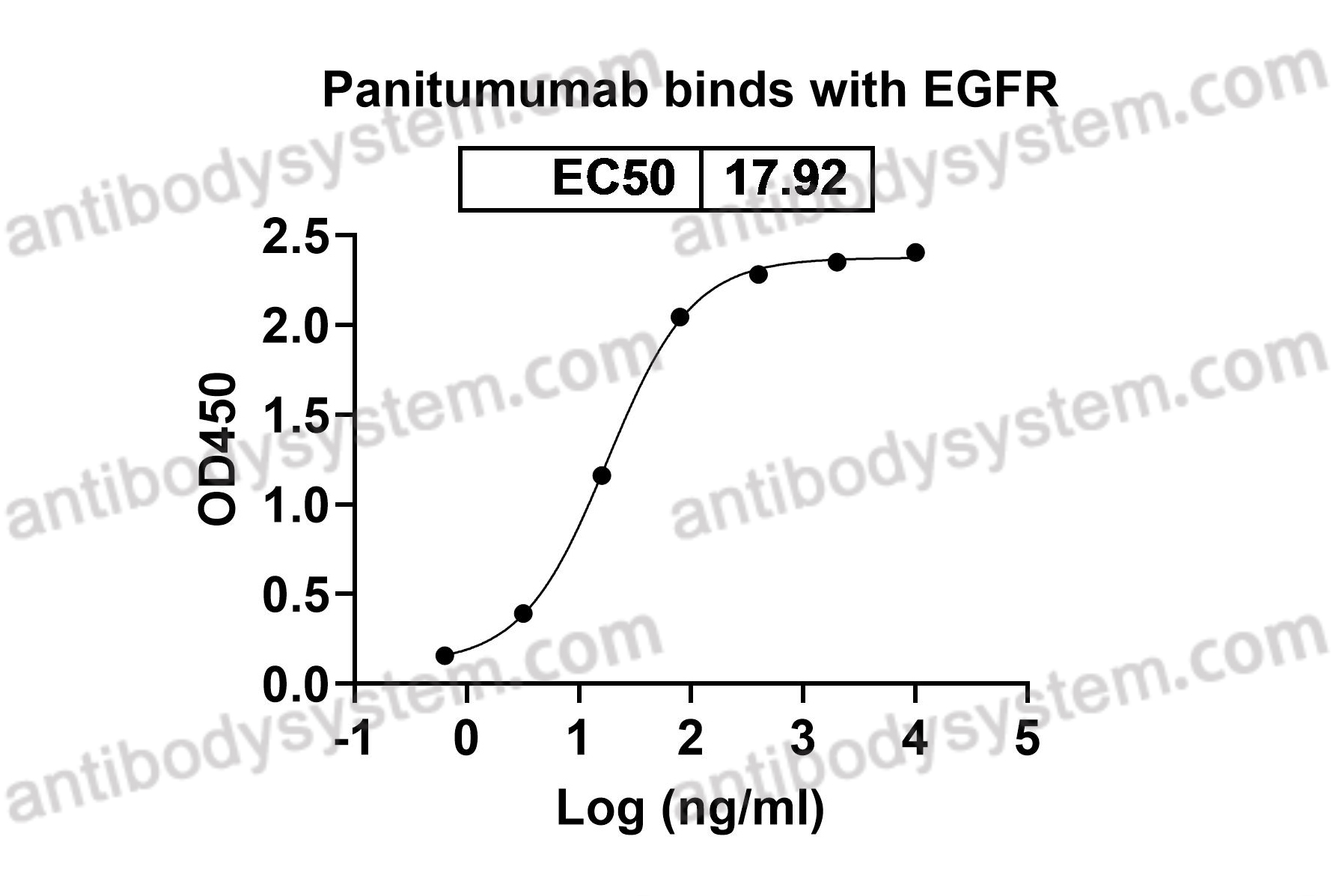
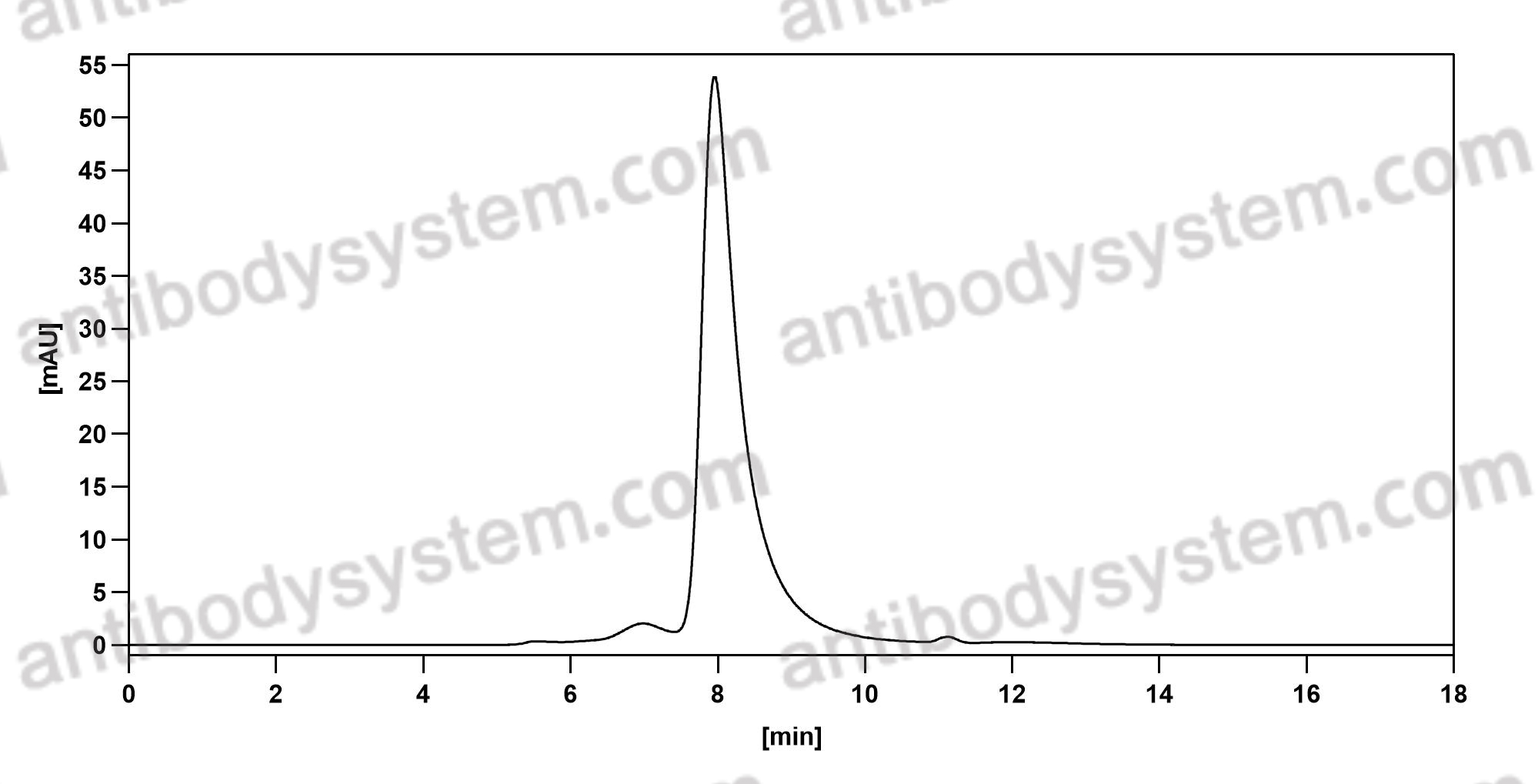
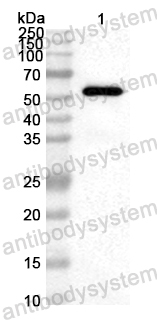
Panitumumab, PMID: 17171190
Different Toxicity of Cetuximab and Panitumumab in Metastatic Colorectal Cancer Treatment: A Systematic Review and Meta-Analysis, PMID: 29393280
FOLFOXIRI Plus Panitumumab As First-Line Treatment of RAS Wild-Type Metastatic Colorectal Cancer: The Randomized, Open-Label, Phase II VOLFI Study (AIO KRK0109), PMID: 31609637
Randomised phase II study of panitumumab plus irinotecan versus cetuximab plus irinotecan in patients with KRAS wild-type metastatic colorectal cancer refractory to fluoropyrimidine, irinotecan and oxaliplatin (WJOG 6510G), PMID: 32526634
Metastatic colorectal cancer: treatment with panitumumab, PMID: 30365654
Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer, PMID: 18316791
Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study, PMID: 24739896
Panitumumab in the treatment of metastatic colorectal cancer, including wild-type RAS, KRAS and NRAS mCRC, PMID: 29737864
FOLFOXIRI-Bevacizumab or FOLFOX-Panitumumab in Patients with Left-Sided RAS/BRAF Wild-Type Metastatic Colorectal Cancer: A Propensity Score-Based Analysis, PMID: 33336844
Negative Hyperselection of Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer Who Received Panitumumab-Based Maintenance Therapy, PMID: 31539295
Panitumumab-induced pulmonary toxicity, PMID: 31708664
Trichomegaly Associated with Panitumumab, PMID: 33053288
Panitumumab, PMID: 19843050
Panitumumab, PMID: 17201026
Panitumumab in metastatic colorectal cancer, PMID: 23802848
Panitumumab safety for treating colorectal cancer, PMID: 24766434
Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma, PMID: 28424871
Panitumumab for the treatment of metastatic colorectal cancer: a review, PMID: 26250414
Panitumumab: in the treatment of metastatic colorectal cancer, PMID: 17100412
Panitumumab (vectibix), PMID: 21596817
Panitumumab in combination with cytotoxic chemotherapy for the treatment of metastatic colorectal carcinoma, PMID: 21925954
Panitumumab: in metastatic colorectal cancer with wild-type KRAS, PMID: 18998757
Panitumumab in the treatment of colon cancer: A biomarker dilemma, PMID: 25374966
The efficacy of panitumumab in refractory metastatic colorectal cancer: A meta-analysis, PMID: 31646791
Panitumumab use in metastatic colorectal cancer and patterns of RAS testing: results from a Europe-wide physician survey and medical records review, PMID: 29183279
Panitumumab-IRDye800CW for Fluorescence-Guided Surgical Resection of Colorectal Cancer, PMID: 30798171
Comparison of Panitumumab-IRDye800CW and 5-Aminolevulinic Acid to Provide Optical Contrast in a Model of Glioblastoma Multiforme, PMID: 32606015
Panitumumab the first fully human monoclonal antibody: from the bench to the clinic, PMID: 17159497
Panitumumab: a summary of clinical development in colorectal cancer and future directions, PMID: 22515440
Spotlight on panitumumab in metastatic colorectal cancer, PMID: 20623992
Comparative Effectiveness and Safety of Monoclonal Antibodies (Bevacizumab, Cetuximab, and Panitumumab) in Combination with Chemotherapy for Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis, PMID: 30499082
Panitumumab: a fully human monoclonal antibody with activity in metastatic colorectal cancer, PMID: 17355997
Panitumumab: human monoclonal antibody against epidermal growth factor receptors for the treatment of metastatic colorectal cancer, PMID: 18343240
Panitumumab in Metastatic Colorectal Cancer: The Importance of Tumour RAS Status, PMID: 25895463
Panitumumab : leading to better overall survival in metastatic colorectal cancer?, PMID: 24593265
Panitumumab Modified with Metal-Chelating Polymers (MCP) Complexed to 111 In and 177 Lu-An EGFR-Targeted Theranostic for Pancreatic Cancer, PMID: 29314858
Panitumumab: A Review of Clinical Pharmacokinetic and Pharmacology Properties After Over a Decade of Experience in Patients with Solid Tumors, PMID: 34152568
Panitumumab-based maintenance after oxaliplatin discontinuation in metastatic colorectal cancer: A retrospective analysis of two randomised trials, PMID: 30614531
Cabozantinib and Panitumumab for RAS Wild-Type Metastatic Colorectal Cancer, PMID: 33469991
Role of panitumumab in the management of metastatic colorectal cancer, PMID: 16945167
Panitumumab in colon cancer: a review and summary of ongoing trials, PMID: 17049019
Clinical Pharmacokinetics and Pharmacodynamics of the Epidermal Growth Factor Receptor Inhibitor Panitumumab in the Treatment of Colorectal Cancer, PMID: 28853050
KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer - practical implications for the clinician, PMID: 31553708
Pharmacokinetic and pharmacodynamic perspectives on the clinical drug development of panitumumab, PMID: 20923247
The efficacy and safety of panitumumab supplementation for colorectal cancer: A meta-analysis of randomized controlled studies, PMID: 32176047
Panitumumab-Conjugated and Platinum-Cored pH-Sensitive Apoferritin Nanocages for Colorectal Cancer-Targeted Therapy, PMID: 29368506
Panitumumab - an effective long-term treatment for patients with metastatic colorectal cancer and wild-type KRAS status, PMID: 20189055
Phase II Study of Panitumumab Monotherapy in Chemotherapy-Naïve Frail or Elderly Patients with Unresectable RAS Wild-Type Colorectal Cancer: OGSG 1602, PMID: 32918848
Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer, PMID: 29431699
Model-Based Cost-Effectiveness Analysis of Panitumumab Plus FOLFIRI for the Second-Line Treatment of Patients with Wild-Type Ras Metastatic Colorectal Cancer, PMID: 31902066
Case Report WIN-MTB-2023001 WIN International Molecular Tumor Board A 62-year-old male with metastatic colorectal cancer with 5 prior lines of treatment., PMID:40526090
Cost-effectiveness analysis of sotorasib plus panitumumab in the treatment of refractory colorectal cancer with mutated KRAS G12C in the USA., PMID:40518842
Identification of Ion Channel-Associated Prognostic Biomarkers for Lung Adenocarcinoma., PMID:40493900
Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review., PMID:40426729
Exploring Real-World Outcomes of First Line EGFR Inhibitor Use, Cetuximab Versus Panitumumab, in Patients with Left-Sided, RAS Wild-Type, Metastatic Colorectal Cancer., PMID:40413126
Evaluation of the usefulness of protocol-based magnesium supplementation for hypomagnesemia in patients with advanced or recurrent colorectal cancer treated with panitumumab., PMID:40366467
Molecular Characterization and Clinical Outcomes of Pancreatic Neuroendocrine Neoplasms Harboring PAK4-NAMPT Alterations., PMID:40330142
Epidermal growth factor receptor antibody and axial elongation in experimental myopia., PMID:40326259
Construction and evaluation of a novel Stx2a-based immunotoxin against epidermal growth factor receptor (EGFR)., PMID:40315796
Targeting the MAP kinase pathway in colorectal cancer: A journey in personalized medicine., PMID:40310309
KRAS G12C inhibitors as monotherapy or in combination for metastatic colorectal cancer: A proportion and comparative meta-analysis of efficacy and toxicity from phase I-II-III trials., PMID:40274247
Efficacy and safety of oxaliplatin-based chemotherapy as first-line treatment in elderly patients with metastatic colorectal cancer: a meta-analysis., PMID:40260292
Overall Survival Analysis of the Phase III CodeBreaK 300 Study of Sotorasib Plus Panitumumab Versus Investigator's Choice in Chemorefractory KRAS G12C Colorectal Cancer., PMID:40215429
[A Case of Long-Term Prognosis of Advanced Rectosigmoid Cancer with Multiple Metastases Treated with Multidisciplinary Therapy]., PMID:40189763
Black Pigmentation on the Tongue Induced by Long-Term Use of Tetracycline Antibiotics in a Colorectal Cancer Patient With Epidermal Growth Factor Receptor (EGFR) Inhibitor-Associated Skin Lesions: A Case Report and Literature Review., PMID:40130130
Colorectal Cancer: Risk Factors, Novel Approaches in Molecular Screening and Treatment., PMID:40123590
Efficacy and safety of KRAS -G12C inhibitors in colorectal cancer: a systematic review of clinical trials., PMID:40116975
Response to anti-EGFR therapy in chemo-refractory right-sided RAS wild-type metastatic colorectal cancer: a case report and literature review., PMID:40115908
Background Tissue with Native Target Expression Can Determine Presence of Nodal Metastasis in Head and Neck Squamous Cell Carcinoma Patients Infused with Targeted Fluorescent Tracers., PMID:40100567
Treatment of extended RAS/BRAF wild-type metastatic colorectal cancer with anti-EGFR antibody combinations., PMID:40097366
HER2-targeted therapy in colorectal cancer: a comprehensive review., PMID:40087250
The emerging role of Sotorasib plus Panitumumab combination therapy in colorectal cancer treatment., PMID:40080361
Identifying distinct prognostic and predictive contributions of tumor epithelium versus tumor microenvironment in colorectal cancer., PMID:40075322
Costs of Adverse Event Management Associated with First-Line Cetuximab or Panitumumab in Metastatic Colorectal Cancer Patients in Saudi Arabia., PMID:40061835
Molecular Markers of Occult Lymph Node Metastasis in Head and Neck Squamous Cell Carcinoma (HNSCC) Patients., PMID:40018925
Aphthous-Like Stomatitis in a Patient Receiving Panitumumab., PMID:40017685
The Pharmacologic Inhibition of KRAS Mutants as a Treatment for Cancer: Therapeutic Principles and Clinical Results., PMID:40009739
Real-World Evidence of Bevacizumab and Panitumumab Drug Resistance and Drug Ineffectiveness from EudraVigilance Database., PMID:40002260
[A Case of Colorectal Cancer with Multiple Unresectable Liver Metastases That Achieved R0 after Chemotherapy and Laparoscopic Two-Stage Hepatectomy]., PMID:39949006
Could Panitumumab with very low dose Capecitabine be an option as a maintenance regimen., PMID:39945465
Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C., PMID:39938112
Results of the Phase I/II Study and Preliminary B-cell Gene Signature of Combined Inhibition of Glutamine Metabolism and EGFR in Colorectal Cancer., PMID:39927885
Using methods to extend inferences to specific target populations to improve the precision of subgroup analyses., PMID:39924128
Monitoring of occupational exposure to hazardous medicinal products in robotic compounding., PMID:39890433
Beyond chemotherapy: Exploring 5-FU resistance and stemness in colorectal cancer., PMID:39863147
An Exosome-Based Liquid Biopsy Predicts Depth of Response and Survival Outcomes to Cetuximab and Panitumumab in Metastatic Colorectal Cancer: The EXONERATE Study., PMID:39820673
Neo-adjuvant FOLFOX with and without panitumumab for patients with KRAS-wt locally advanced colon cancer: results following an extended biomarker panel on the FOxTROT trial embedded phase II population., PMID:39805350
Panitumumab plus 5-fluorouracil and folinic acid or 5-fluorouracil and folinic acid alone as maintenance therapy in RAS wild-type metastatic colorectal cancer (PanaMa, AIO KRK 0212): final efficacy analysis of a randomised, open-label, phase 2 trial., PMID:39802302
Panitumumab versus cetuximab in combination with irinotecan in refractory metastatic colorectal cancer., PMID:39799855
AGITG MONARCC: A Randomized Phase 2 Study of Panitumumab Monotherapy and Panitumumab Plus 5-Fluorouracil as First-Line Therapy for Older Patients With RAS and BRAF Wild Type Metastatic Colorectal Cancer. A Study by the Australasian Gastro-Intestinal Trials Group (AGITG)., PMID:39779412
Au@109Pd Core-Shell Nanoparticles Conjugated to Panitumumab for the Combined β--Auger Electron Therapy of Triple-Negative Breast Cancer., PMID:39769315
Development of KRAS Inhibitors and Their Role for Metastatic Colorectal Cancer., PMID:39752885
Limited Efficacy of Anti-EGFR Monoclonal Antibodies in Colorectal Cancer Patients with Rare RAS Variants: Analysis of the C-CAT Database., PMID:39727997
[Case of Minocycline-Induced Thrombocytopenia during Chemotherapy for Ascending Colon Cancer]., PMID:39721793
The efficacy of targeted therapy and/or immunotherapy with or without chemotherapy in patients with colorectal cancer: A network meta-analysis., PMID:39716565
Second-line systemic treatment for metastatic colorectal cancer: A systematic review and Bayesian network meta-analysis based on RCT., PMID:39715232
Circulating Hepcidin Levels Are an Independent Predictor of Survival in Microsatellite Stable Metastatic Colorectal Cancer Patient Candidates for Standard First-Line Treatment., PMID:39682164
Prognostic value of radiologic and pathological response in colorectal cancer liver metastases upon systemic induction treatment: subgroup analysis of the CAIRO5 trial., PMID:39667310
Intensified Total Neoadjuvant Therapy in Patients With Locally Advanced Rectal Cancer: Long-term Results of a Prospective Phase II Study., PMID:39667262
Post-marketing safety of panitumumab: a real-world pharmacovigilance study., PMID:39651795