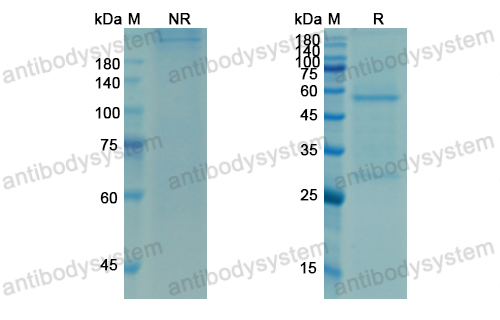
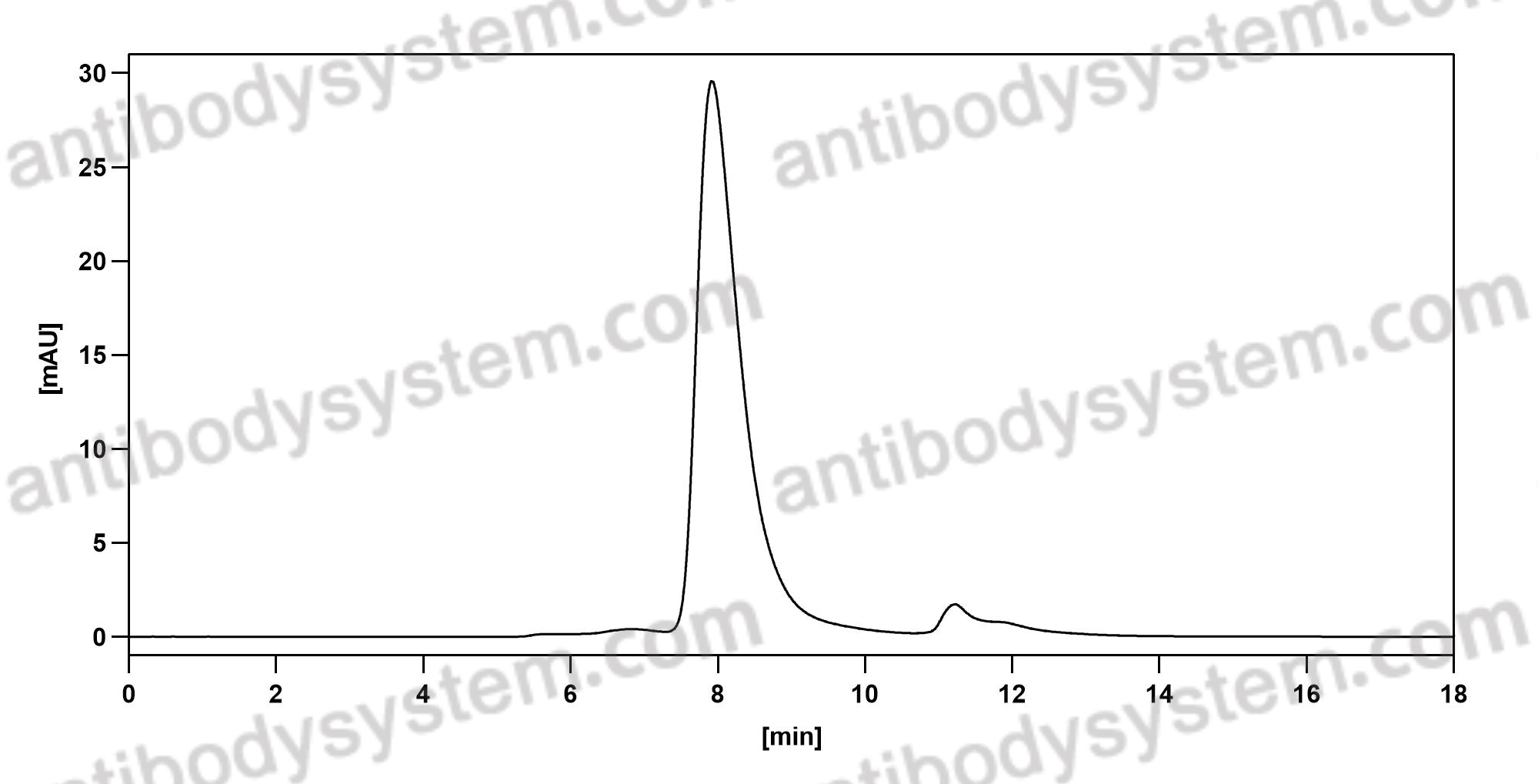
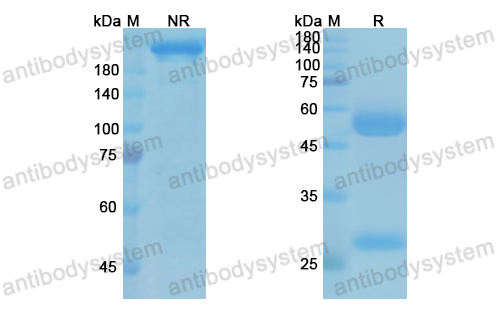
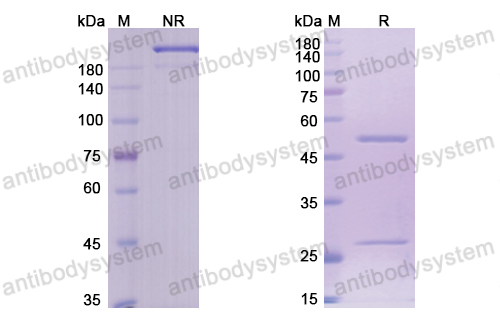
Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials, PMID: 32524991
Roles of omalizumab in various allergic diseases, PMID: 32067933
The use of omalizumab in allergen immunotherapy, PMID: 29315922
Omalizumab Updosing in Chronic Spontaneous Urticaria: an Overview of Real-World Evidence, PMID: 32418171
Omalizumab in the Treatment of Chronic Inducible Urticaria, PMID: 27717421
From IgE to Omalizumab, PMID: 27864548
Omalizumab, the first available antibody for biological treatment of severe asthma: more than a decade of real-life effectiveness, PMID: 30400762
Off-Label Uses of Omalizumab, PMID: 26048266
Omalizumab for atopic dermatitis: evidence for and against its use, PMID: 30717578
Omalizumab for Severe Asthma: Beyond Allergic Asthma, PMID: 30310816
Omalizumab for chronic urticaria in children younger than 12 years, PMID: 31082483
Benefits and Harms of Omalizumab Treatment in Adolescent and Adult Patients With Chronic Idiopathic (Spontaneous) Urticaria: A Meta-analysis of "Real-world" Evidence, PMID: 30427977
Omalizumab for the Treatment of Chronic Idiopathic Urticaria: Systematic Review of the Literature, PMID: 28226418
Efficacy and safety of omalizumab in children and adolescents with moderate-to-severe asthma: A systematic literature review, PMID: 28631599
Efficacy and effectiveness of omalizumab in the treatment of childhood asthma, PMID: 30141696
The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma, PMID: 31049972
Omalizumab in children with uncontrolled allergic asthma: Review of clinical trial and real-world experience, PMID: 28477722
Effect of omalizumab on lung function and eosinophil levels in adolescents with moderate-to-severe allergic asthma, PMID: 31760132
Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence, PMID: 28751232
Rituximab and Omalizumab for the Treatment of Bullous Pemphigoid: A Systematic Review of the Literature, PMID: 30421306
The anti-IgE mAb omalizumab induces adverse reactions by engaging Fcγ receptors, PMID: 31770111
Omalizumab in children and adolescents with chronic spontaneous urticaria: Case series and review of the literature, PMID: 32358910
How to Treat Patients with Chronic Spontaneous Urticaria with Omalizumab: Questions and Answers, PMID: 31374358
Mabs for treating asthma: omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, PMID: 32401603
Pediatric use of omalizumab for allergic asthma, PMID: 32241196
Omalizumab in severe eczema, PMID: 32725741
Omalizumab for Atopic Dermatitis: Overtreatment or Lifesaver?, PMID: 31764954
Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: A long-term post-marketing study in Japan, PMID: 30053973
[Omalizumab: Beyond anti-IgE properties], PMID: 28189435
Omalizumab retreatment in patients with chronic spontaneous urticaria: a systematic review of published evidence, PMID: 32108461
Should omalizumab be used in severe asthma/COPD overlap?, PMID: 30043557
The XTEND-CIU study: Long-term use of omalizumab in chronic idiopathic urticaria, PMID: 29132956
Novel targets of omalizumab in asthma, PMID: 27798419
Role of Biologics in Asthma, PMID: 30525902
Omalizumab re-treatment rates in chronic spontaneous urticaria, PMID: 32378397
Omalizumab: living up to PROMises, PMID: 30604545
The discovery and development of omalizumab for the treatment of asthma, PMID: 25979110
Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: systematic review of 42 studies published 2008-2018, PMID: 30763137
New insights into the utility of omalizumab, PMID: 30690050
A review of omalizumab for the management of severe asthma, PMID: 27528798
Omalizumab Is an Effective Intervention in Severe Asthma with Fungal Sensitization, PMID: 32561499
Omalizumab and mepolizumab in the landscape of biological therapy for severe asthma in children: how to choose?, PMID: 31779657
Omalizumab and Mast Cell Disorders: Are We There Yet?, PMID: 31495435
The Use of Omalizumab in Food Oral Immunotherapy, PMID: 27628022
Algorithm for Treatment of Chronic Spontaneous Urticaria with Omalizumab, PMID: 30107875
Response of omalizumab in normocomplementemic urticarial vasculitis, PMID: 32145404
Omalizumab treatment in adolescents with chronic spontaneous urticaria: Efficacy and safety, PMID: 32467066
Omalizumab concentrations in pregnancy and lactation: A case study, PMID: 32544544
Update on Omalizumab for Urticaria: What's New in the Literature from Mechanisms to Clinic, PMID: 29744661
Omalizumab or dupilumab for chronic rhinosinusitis with nasal polyposis, PMID: 33160644
Cold urticaria patients can be susceptible to anaphylaxis during diagnostic cold provocation tests., PMID:40527541
Describing Clinical Characteristics and Treatment Course of Patients with Hereditary Alpha-tryptasemia: A Single-center Study., PMID:40515774
Biologics in IgE-mediated food allergy: A systematic review and meta-analysis of interventional studies., PMID:40510735
Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Immunogenicity Profiles of a Single-Dose JYB1904 in Healthy Chinese Participants: A Phase Ia, Randomized, Double-Blind, Placebo-Controlled Study., PMID:40504164
Health care resource utilization of patients with asthma and food allergy initiating omalizumab., PMID:40497014
Corrigendum to "Omalizumab may protect allergic patients against COVID-19: A systematic review" [World Allergy Organ J 16 (2) (2023) 1-19]., PMID:40496753
Comparative Analysis of ICS Combined with LABA versus Addition of Omalizumab on Transcriptomic Expression Profiles in Patients with Allergic Asthma., PMID:40491983
Aspirin-Exacerbated Respiratory Disease in the era of biologics., PMID:40490219
Advancements in Biologic Therapies for Pediatric Asthma: Emerging Therapies and Future Directions., PMID:40486460
Identification of the Top 15 Drugs Associated With Anaphylaxis: A Pharmacovigilance Study., PMID:40484712
A nationwide experience of biological treatments in children with eosinophilic esophagitis., PMID:40483369
How to manage hypersensitivity reactions to enzyme replacement therapy in lysosomal storage diseases?, PMID:40481554
Prognostic calculator of the clinical response to antihistamines in chronic urticaria: External validation., PMID:40480548
Azathioprine for the Treatment of Chronic Spontaneous Urticaria Refractory to Antihistamines, Omalizumab, and Ciclosporin: A Case Series., PMID:40480489
Equivalence of SYN008 versus omalizumab in patients with refractory chronic spontaneous urticaria: A multicenter, randomized, double-blind, parallel-group, active-controlled phase III study., PMID:40474341
The Efficacy of Different Dosing Regimens of Omalizumab in children and adolescents with chronic spontaneous urticaria based on real-life data., PMID:40466628
In brief: Omlyclo - an omalizumab biosimilar interchangeable with Xolair., PMID:40459407
Evaluating remibrutinib in the treatment of chronic spontaneous urticaria., PMID:40455080
Efficacy and safety of CT-P39, an omalizumab biosimilar, in chronic spontaneous urticaria: 16-week follow-up study., PMID:40454995
Hereditary alpha-tryptasemia - a potential cause of severe anaphylactic reactions and a modifier of mast cell diseases., PMID:40450750
Introduction of Allergenic Foods After Treatment with Omalizumab., PMID:40447195
Efficacy and safety of Omalizumab in the treatment of CSU: a meta-analysis of a randomized controlled trial., PMID:40439896
Omalizumab Efficacy in Chronic Rhinosinusitis Patients with Recurrent Nasal Polyps: An open-label, single-center, randomized, controlled study., PMID:40437843
Omalizumab in Food Allergy in Children: Current Evidence and Future Perspectives., PMID:40430110
Four-week IgE/baseline IgE ratio combined with tryptase predicts clinical outcome in omalizumab-treated children with moderate-to-severe asthma., PMID:40417314
Anti-KIT Barzolvolimab for Chronic Spontaneous Urticaria., PMID:40415544
Treatment of eosinophilic dermatosis of hematologic malignancy with omalizumab., PMID:40413842
Redefining Omalizumab Discontinuation in Chronic Spontaneous Urticaria: The Value of Optimization and Predictive Factors of Relapse. A 52-Week Multicenter Study., PMID:40403841
Bioinformatics analysis of FCER1A as a key immune marker in dilated cardiomyopathy and systemic lupus erythematosus., PMID:40401008
Both Extrinsic and Intrinsic Atopy in Chronic Spontaneous Urticaria Patients Indicate a Favorable Response to Omalizumab Treatment., PMID:40397623
Resolution of Patient's Anaphylaxis to the Moderna COVID-19 Vaccine by Desensitization., PMID:40386747
Anaphylaxis in children: Latest insights., PMID:40380371
Value-based care in allergy-immunology: Beyond the quality-adjusted life year., PMID:40380361
Emerging Therapies in the Treatment of Prurigo Nodularis: Biological Therapy and Systematic Review of Literature., PMID:40372666
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Omalizumab dosing patterns and drug survival in adult patients with chronic spontaneous urticaria., PMID:40357715
Combination of Allergen-Specific Immunotherapy With Biologics in Severe Asthma: Counterintuitive or Rational?, PMID:40349962
Requalification of patients with severe asthma for biological therapy-Practical 'ReQuaBi' rate decision scheme based on the analytical model., PMID:40348613
Reappraisal of Biologic Efficacy from Phase 3 Trials in Refractory Chronic Rhinosinusitis and Nasal Polyps., PMID:40340024
Omalizumab: a broader role in dermatology? Evidence and the road ahead., PMID:40338196
Pityriasis rosea-like eruption induced by omalizumab: a case report of a rare side effect., PMID:40330944
Scientific developments in understanding food allergy prevention, diagnosis, and treatment., PMID:40330465
Biological and target synthetic treatments for chronic spontaneous urticaria: A systematic review and network meta-analysis., PMID:40326848
Spectrum and Impact of Reported Side Effects of Omalizumab in Patients With Chronic Urticaria: A Long-Term Multicentre Real-World Study., PMID:40325793
Glycobiology of IgE., PMID:40322856
Recurrent Chronic Idiopathic Urticaria Post-hysterectomy and Loss of Xolair Effectiveness: A Case Report., PMID:40322383
Structural and Functional Insights Into IgE Receptor Interactions and Disruptive Inhibition., PMID:40305523
Efficacy of tiotropium bromide on spirometric measurements and control of asthma in real life: data from a 1-year clinical follow-up., PMID:40304436
Mental Health Interventions in Refractory Chronic Spontaneous Urticaria: A Call to Expand Treatment Guidelines., PMID:40303526
Secukinumab as a Novel Treatment for Chronic Netherton Syndrome in a Young Adult., PMID:40299794