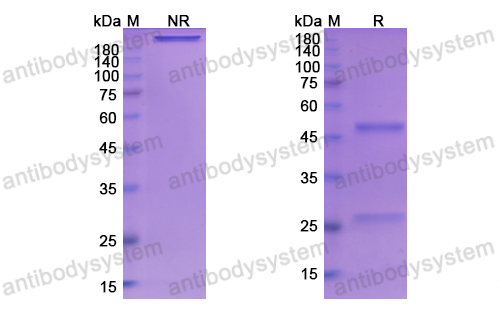
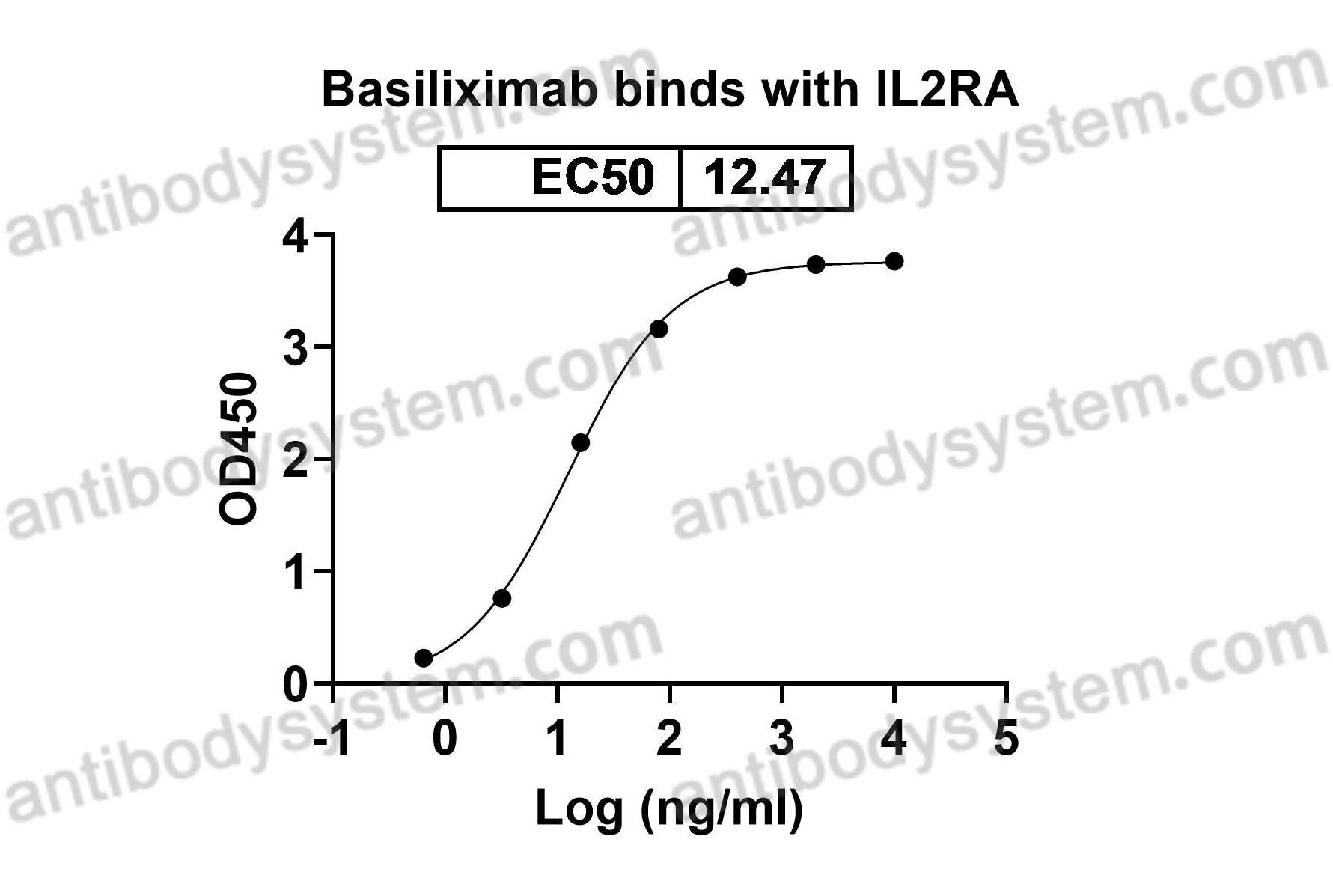
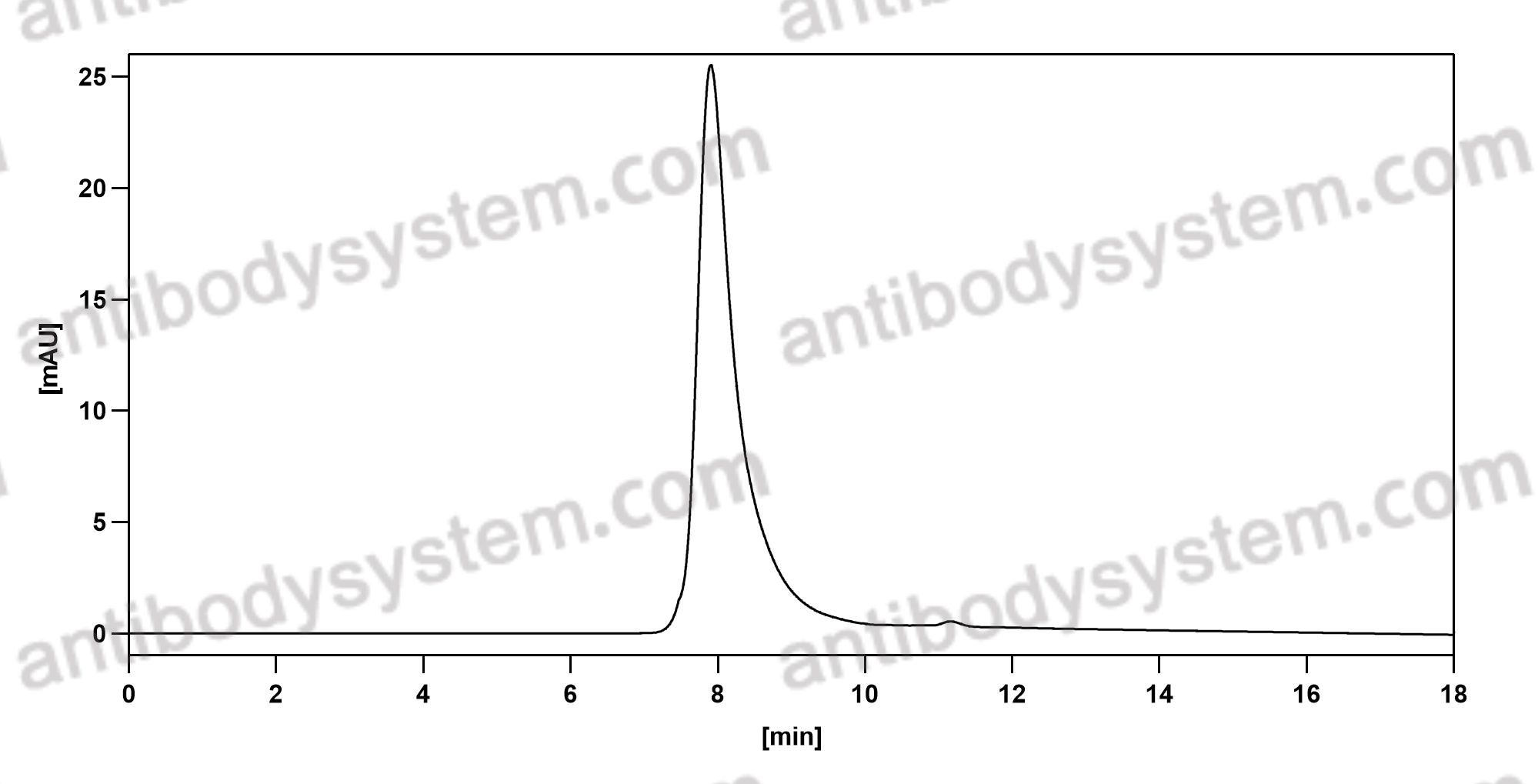
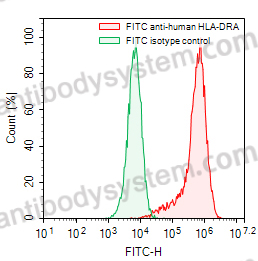
Basiliximab, PMID: 10188761
Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open-label, multicentre, randomised controlled trial, PMID: 27871759
Basiliximab: a review of its use as induction therapy in renal transplantation, PMID: 14664658
Induction therapy of basiliximab versus antithymocyte globulin in renal allograft: a systematic review and meta-analysis, PMID: 28986715
A benefit-risk assessment of basiliximab in renal transplantation, PMID: 14717621
Efficacy and safety of basiliximab in kidney transplantation, PMID: 15934854
Does basiliximab induction trigger lifethreatening ARDS and shock in young patients after kidney transplantation?, PMID: 24131675
Low-dose Thymoglobulin vs Basiliximab Induction Therapy in Low-Risk Living Related Kidney Transplant Recipients: A Prospective Randomized Trial, PMID: 32178925
Basiliximab, mechanism of action and pharmacological properties, PMID: 15648237
Biologic therapy in the idiopathic inflammatory myopathies, PMID: 31680207
Efficacy and safety of basiliximab as initial immunosuppression in liver transplantation: A single center study, PMID: 32768592
Basiliximab as Treatment for Steroid-Refractory Acute Graft-versus-Host Disease in Pediatric Patients after Haploidentical Hematopoietic Stem Cell Transplantation, PMID: 31704470
Pharmacokinetics of Basiliximab for the Prevention of Graft-versus-Host Disease in Patients Undergoing Hematopoietic Cell Transplantation with Minimal-Intensity Cyclophosphamide and Fludarabine, PMID: 31742732
The use of basiliximab in solid organ transplantation, PMID: 12437498
Comparison of Alemtuzumab Versus Basiliximab Induction Therapy in Elderly Kidney Transplant Recipients: A Single-Center Experience, PMID: 31315501
The role of basiliximab induction therapy in organ transplantation, PMID: 17150025
Basiliximab treatment of severe GVHD induced by donor lymphocyte infusion with interferon-a, PMID: 30589491
Early Steroid Withdrawal Protocol With Basiliximab and Rituximab in ABO-Incompatible Kidney Transplant Recipients, PMID: 32444132
Prognostic factors and long-term follow-up of basiliximab for steroid-refractory acute graft-versus-host disease: Updated experience from a large-scale study, PMID: 32311156
Clinical-economic impact of the change of protocol for use of basiliximab in liver transplant, PMID: 30624168
Opportunistic Infections After Induction With Alemtuzumab or Basiliximab: A 3-Year Kidney Transplantation Experience, PMID: 32921434
Basiliximab impairs regulatory T cell (TREG) function and could affect the short-term graft acceptance in children with heart transplantation, PMID: 33436905
Immunosuppression and Reproductive Health After Kidney Transplantation, PMID: 31397802
Thymoglobuline plus basiliximab a mixed cocktail to start?, PMID: 28676335
Anti-interleukin-2 receptor antibodies: basiliximab and daclizumab, PMID: 11522853
Comparison of basiliximab vs antithymocyte globulin for induction in pediatric heart transplant recipients: An analysis of the International Society for Heart and Lung Transplantation database, PMID: 29878688
Basiliximab: efficacy and safety evaluation in kidney transplantation, PMID: 24266670
Basiliximab for posterior reversible encephalopathy syndrome after lung transplantation, PMID: 28498905
Basiliximab induction in patients receiving tacrolimus-based immunosuppressive regimens, PMID: 23054318
Economic analysis of basiliximab in renal transplantation, PMID: 11435967
Basiliximab does not increase efficacy of corticosteroids in patients with steroid-refractory ulcerative colitis, PMID: 22549092
Low-dose basiliximab induction therapy in heart transplantation, PMID: 28990220
Thymoglobulin Versus Basiliximab Induction Therapy in Low-Risk Kidney Transplant Recipients: A Single-Center Experience, PMID: 29880348
Induction Therapy With ATG Compared With Anti-IL2 Basiliximab in Low-Immunologic Risk Kidney Transplant Recipients, PMID: 31732198
Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: a meta-analysis, PMID: 20620496
[Basiliximab following penetrating risk-keratoplasty--a prospective randomized pilot study], PMID: 18236372
Early reduction of regulatory T cells is associated with acute rejection in liver transplantation under tacrolimus-based immunosuppression with basiliximab induction, PMID: 31965710
Assessment of infectious complications in elderly kidney transplant recipients receiving induction with anti-thymocyte globulin vs basiliximab, PMID: 32031729
Use of basiliximab in pediatric liver transplantation for Langerhans cell histiocytosis, PMID: 12756053
A prospective economic evaluation of basiliximab (Simulect) therapy following renal transplantation, PMID: 11048993
[Off-label use of biologic agents in the treatment of dermatosis, part 2: etanercept, efalizumab, alefacept, rituximab, daclizumab, basiliximab, omalizumab, and cetuximab], PMID: 18206084
Basiliximab (Simulect) in renal transplantation with high risk for delayed graft function, PMID: 15866629
Basiliximab in pediatric liver transplantation: a pharmacokinetic-derived dosing algorithm, PMID: 12100507
Single-Dose Basiliximab Induction in Low-Risk Renal Transplant Recipients, PMID: 27265620
Basiliximab and heart transplantation in Hispanics: the experience in Puerto Rico, PMID: 19610574
Increased risk of rejection after basiliximab induction in sensitized kidney transplant recipients without pre-existing donor-specific antibodies - a retrospective study, PMID: 30903722
Basiliximab-chimeric anti-IL2-R monoclonal antibody in pediatric liver transplantation: comparative study, PMID: 15194332
Basiliximab induction and delayed calcineurin inhibitor initiation in liver transplant recipients with renal insufficiency, PMID: 21617588
Analysis of rabbit anti-thymocyte globulin vs basiliximab induction in pediatric liver transplant recipients, PMID: 31512802
Basiliximab in lung transplantation: preliminary experience, PMID: 15866665
Thrombotic Microangiopathy After Kidney Pancreas Transplant Managed With Eculizumab and a Calcineurin Inhibitor-free Basiliximab/Belatacept Maintenance Regimen: Between a Rock and a Hard Place., PMID:40519672
Induction With Antithymocyte Globulin Is Associated With Decreased Mortality and PTLD in Pediatric Liver Transplantation: A UNOS Data Analysis., PMID:40490923
TIM3 and TIGIT-expressing CD4 T cells are impacted by kidney transplantation and associated with risk of infection., PMID:40475763
Incidence of catheter-related bloodstream infection (CRBSI) in immunosuppressed hosts post solid organ transplant (SOT): a single center experience., PMID:40438131
Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study., PMID:40429403
The first live term birth following uterus transplantation in Australia., PMID:40406819
Infection Risks With Thymoglobulin Use for Delayed Graft Function in Deceased Donor Kidney Transplantation: Research Letter., PMID:40352746
Efficacy and Safety of Induction Therapy in Kidney Transplant Recipients: A Propensity Score Matching Analysis in a Multicenter Retrospective Observational Study., PMID:40312217
Optimization of the Tacrolimus Concentration-to-Dose Ratio Cut-Off Value to Define Metabolism Groups., PMID:40283373
Machine Learning Model for Predicting Biliary Complications After Liver Transplantation., PMID:40249076
Evaluation of biological therapies in autoimmune hepatitis: A case-based systematic review., PMID:40123748
Basiliximab for IL-2-Associated Inflammatory Disorder With Atopic Dermatitis., PMID:40000115
Acute Respiratory Distress Syndrome in Young Postrenal Transplant Patients Receiving Basiliximab., PMID:39996094
Evaluating the efficacy of basiliximab versus no induction in low-immunological-risk kidney transplant recipients: a propensity score matched analysis., PMID:39978365
Induction outcomes in adult kidney transplantation: Two decades of UNOS data analysis., PMID:39947488
Patterns of belatacept use and risk of post-transplant lymphoproliferative disorder in US kidney transplant recipients: An analysis of the Organ Procurement and Transplantation Network database., PMID:39792912
Association Between Early Immunosuppression Center Variability and One-Year Outcomes After Pediatric Liver Transplant., PMID:39777775
Assessment of respiratory function in children after kidney transplantation., PMID:39775938
Identifying Promising Immunomodulators for Type 1 Diabetes (T1D) and Islet Transplantation., PMID:39735417
Rejection in the setting of combined Heart and Liver Transplantation., PMID:39713488
Alemtuzumab Associated With Higher Mortality Than Basiliximab in Older Kidney Transplant Recipients., PMID:39708389
The Use of Long-Term Monthly Basiliximab Infusions as Rescue Maintenance Immunosuppression in Pancreas Transplant Recipients., PMID:39688531
First ABO-Incompatible Pediatric Kidney Transplant in South Africa-A Case Report., PMID:39688284
Combined Cytokine Blockade Therapy (CCBT) Using Basiliximab and Infliximab for Treatment of Steroid-Refractory Graft-Versus-Host Disease (SR-GvHD)., PMID:39682101
Advancing kidney transplantation in black patients: a genetics-based and personalized approach under NICE, KDIGO, and ERBP guidelines., PMID:39676231
Basiliximab is superior to low dose rabbit anti-thymocyte globulin in pediatric kidney transplant recipients: The younger, the better., PMID:39658242
Basiliximab induction immunosuppression and lung transplant outcomes: Propensity analysis in a multicenter cohort., PMID:39642952
The role of induction therapy in lung transplantation., PMID:39551266
Retrospective Cohort Study of Associated Factors for Intestinal Complications in Pediatric Liver Transplantation., PMID:39526469
Beyond Borders: International Immunosuppression Practices in Pediatric Lung Transplantation., PMID:39520098
Pediatric kidney transplantation in Europe, a clinical snapshot pilot., PMID:39513158
European Association of Urology Guidelines on Renal Transplantation: Update 2024., PMID:39489684
Basiliximab vs. No Induction Therapy in Kidney Transplant Recipients with a Low Immunological Risk Profile Receiving Tacrolimus/Mycophenolate/Steroids Maintenance Immunosuppression., PMID:39458101
Evaluation of Single Versus Two-Dose Basiliximab Induction Therapy in Live-Donor Liver Transplant., PMID:39436115
Haploidentical hematopoietic stem cell transplantation for hematologic malignancies: a novel conditioning regimen with anti-T lymphocyte immunoglobulin instead of anti-thymocyte globulin for in vivo T cell depletion., PMID:39402188
Clinical profile and outcome of kidney transplantation at Muhimbili National Hospital, Tanzania., PMID:39342167
Basiliximab induction alone vs a dual ATG-basiliximab approach in first live-donor non-sensitized kidney transplant recipients with low HLA matching., PMID:39314868
Intraoperative Mean Arterial Pressure and Postoperative Delayed Graft Function in Kidney Transplantation: Evaluating Three Commonly Used Thresholds., PMID:39302234
Safety assessment of basiliximab using real-world adverse event data from the FDA Adverse Event Reporting System Database: A retrospective observational study., PMID:39252278
Post-Kidney Transplant Cancer: A Real-World Retrospective Analysis From a Single Italian Center., PMID:39228659
Reversible cerebral vasoconstriction syndrome post-cardiac transplantation: a therapeutic dilemma: case report., PMID:39123195
Early acute kidney injury after tacrolimus administration in heart transplant recipients receiving basiliximab induction therapy., PMID:39120080
Role of Induction in a Haplomatch, Related, Low-Risk, Living-Donor Kidney Transplantation with Triple Drug Immunosuppression: A Single-Center Study., PMID:39114397
Contemporary Immunosuppression Management and 1-Year Outcomes in Dual Organ Heart Transplantation., PMID:39113661
Low-Dose Thymoglobulin versus Basiliximab Induction Therapy in Low-Risk Living Related Kidney Transplant Recipients: Three-Year Follow-Up Study., PMID:39079480
Efficacy and Safety of Bleselumab in Preventing the Recurrence of Primary Focal Segmental Glomerulosclerosis in Kidney Transplant Recipients: A Phase 2a, Randomized, Multicenter Study., PMID:39042770
Vedolizumab plus basiliximab as second-line therapy for steroid-refractory lower gastrointestinal acute graft-versus-host disease., PMID:39021571
Successful A2 to O Simultaneous Liver and Kidney Transplantation in the Setting of Pre-operative Positive HLA Crossmatch: A Case Report., PMID:39004578
Everolimus plus reduced calcineurin inhibitor prevents de novo anti-HLA antibodies and humoral rejection in kidney transplant recipients: 12-month results from the ATHENA study., PMID:38993866
Renal transplantation using kidneys from a donor with high grade cytomegalovirus viraemia: case report and literature review., PMID:38991589