Catalog No.
RVV00310
Expression system
Mammalian Cells
Species reactivity
SARS-CoV-2 (2019-nCoV)
Host species
Human
Isotype
IgG1, kappa
Clonality
Monoclonal
Tested applications
ELISA: 1:4000-1:8000, IHC: 1:50-1:100, WB: 1:2000-1:8000
Target
Spike glycoprotein,S glycoprotein,E2,Peplomer protein,Spike protein S1,S,Receptor-Binding Domain,RBD
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
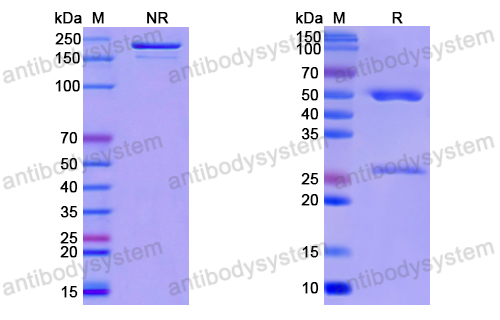
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P0DTC2
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
1A12
The Role of Structural Flexibility in Hydrocarbon-Stapled Peptides Designed to Block Viral Infection via Human ACE2 Mimicry., PMID:39866902
Development of a Recombinant Omicron BA.1 Subunit Vaccine Candidate in Pichia pastoris., PMID:39815667
Impact of variable titer COVID-19 convalescent plasma and recipient SARS-CoV2-specific humoral immunity on survival in hospitalized patients., PMID:39446792
Evaluation of an Affinity-Enhanced Anti-SARS-CoV2 Nanobody Design Workflow Using Machine Learning and Molecular Dynamics., PMID:39356775
Development and characterization of a multimeric recombinant protein using the spike protein receptor binding domain as an antigen to induce SARS-CoV-2 neutralization., PMID:39056544
BCG activation of trained immunity is associated with induction of cross reactive COVID-19 antibodies in a BCG vaccinated population., PMID:38722827
Single-shot dendritic cell targeting SARS-CoV-2 vaccine candidate induces broad, durable and protective systemic and mucosal immunity in mice., PMID:38715364
Safe plant Hsp90 adjuvants elicit an effective immune response against SARS-CoV2-derived RBD antigen., PMID:38631949
Interferon-γ/IL-2 ELISpot and mRNA Responses to the SARS-CoV2, Feline Coronavirus Serotypes 1 (FCoV1), and FCoV2 Receptor Binding Domains by the T Cells from COVID-19-Vaccinated Humans and FCoV1-Infected Cats., PMID:38502392
Molecular insights into the binding interactions and energetics of the omicron spike variant with hACE2 and a neutralizing antibody., PMID:38494148
The effect of dose-interval on antibody response to mRNA COVID-19 vaccines: a prospective cohort study., PMID:38433831
Performance of a Point-of-Care Fluorescence Immunoassay Test to Measure the Anti-Severe Acute Respiratory Syndrome Corona Virus 2 Spike, Receptor Binding Domain Antibody Level., PMID:38132270
Results of the Stop the Spread Ottawa (SSO) cohort study: a Canadian urban-based prospective evaluation of antibody responses and neutralisation efficiency to SARS-CoV-2 infection and vaccination., PMID:37907304
Riding the Wave: Unveiling the Conformational Waves from RBD of SARS-CoV-2 Spike Protein to ACE2., PMID:37769161
Association Between Low Anti-spike Antibody Levels After the Third Dose of SARS-CoV-2 Vaccination and Hospitalization due to Symptomatic Breakthrough Infection in Kidney Transplant Recipients., PMID:37665287
B cell-intrinsic TLR7 signaling is required for neutralizing antibody responses to SARS-CoV-2 and pathogen-like COVID-19 vaccines., PMID:37438976
A nanobody recognizes a unique conserved epitope and potently neutralizes SARS-CoV-2 omicron variants., PMID:37361875
Using a multiplex serological assay to estimate time since SARS-CoV-2 infection and past clinical presentation in malagasy patients., PMID:37332901
Dynamic antibody response in SARS-CoV-2 infected patients and COVID-19 vaccine recipients alongside vaccine effectiveness in comorbid and multimorbid groups., PMID:37251854
Spatially Addressable Multiplex Biodetection by Calibrated Micro/Nanostructured Surfaces., PMID:37099014
Comparative analysis of humoral immune response upon the three first vaccines applied in Argentina: IgG production and neutralizing capacity against SARS-CoV-2., PMID:37090429
In vitro immunogenic profile of recombinant SARS-CoV2 S1-RBD peptide in murine macrophage and microglial cells., PMID:37018795
Third dose of SARS-CoV2 mRNA vaccination produces robust persistent cellular and humoral immune responses in liver transplant recipients., PMID:36929682
Long-term adaptive response in COVID-19 vaccine recipients and the effect of a booster dose., PMID:36926327
Neutralizing Efficacy of Encapsulin Nanoparticles against SARS-CoV2 Variants of Concern., PMID:36851560
Structure-free antibody paratope similarity prediction for in silico epitope binning via protein language models., PMID:36824280
RBD- specific Th1 responses are associated with vaccine-induced protection against SARS-CoV-2 infection in patients with hematological malignancies., PMID:36632566
Evaluation of commercially available fully automated and ELISA-based assays for detecting anti-SARS-CoV-2 neutralizing antibodies., PMID:36347859
Comparing the B and T cell-mediated immune responses in patients with type 2 diabetes receiving mRNA or inactivated COVID-19 vaccines., PMID:36304475
Heterologous SARS-CoV-2 IgA neutralising antibody responses in convalescent plasma., PMID:36299410
Comparison of SARS-CoV-2 Antibody Levels after a Third Heterologous and Homologous BNT162b2 Booster Dose., PMID:36298537
Recombinant expression of SARS-CoV-2 receptor binding domain (RBD) in Escherichia coli and its immunogenicity in mice., PMID:36246069
Serological Response and Clinical Protection of Anti-SARS-CoV-2 Vaccination and the Role of Immunosuppressive Drugs in a Cohort of Kidney Transplant Patients., PMID:36146758
Enhancing antibody responses by multivalent antigen display on thymus-independent DNA origami scaffolds., PMID:36032975
A mosaic-type trimeric RBD-based COVID-19 vaccine candidate induces potent neutralization against Omicron and other SARS-CoV-2 variants., PMID:36004719
Antibody response following the third and fourth SARS-CoV-2 vaccine dose in individuals with common variable immunodeficiency., PMID:35967433
[Investigation of SARS-CoV-2-Specific Humoral and Cellular Immunity Values in Health Care Workers with COVID-19 Disease and Administered with COVID-19 Vaccine]., PMID:35960239
Characterization of a broadly cross reactive tetravalent human monoclonal antibody, recognizing conformational epitopes in receptor binding domain of SARS-CoV-2., PMID:35928502
mRNA Booster Vaccination Enhances Antibody Responses against SARS-CoV2 Omicron Variant in Individuals Primed with mRNA or Inactivated Virus Vaccines., PMID:35891221
Point mutations in SARS-CoV-2 variants induce long-range dynamical perturbations in neutralizing antibodies., PMID:35799828
Application of Baculovirus Expression Vector system (BEV) for COVID-19 diagnostics and therapeutics: a review., PMID:35792966
Ig-VAE: Generative modeling of protein structure by direct 3D coordinate generation., PMID:35759518
Robust anti-SARS-CoV2 single domain antibodies cross neutralize multiple viruses., PMID:35702569
Safety and immunogenicity of the FINLAY-FR-1A vaccine in COVID-19 convalescent participants: an open-label phase 2a and double-blind, randomised, placebo-controlled, phase 2b, seamless, clinical trial., PMID:35691295
Effect of Heterologous Vaccination Strategy on Humoral Response against COVID-19 with CoronaVac plus BNT162b2: A Prospective Cohort Study., PMID:35632443
Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT162b2 vaccine., PMID:35606426
Role of booster with BNT162b2 mRNA in SARS-CoV-2 vaccination in patients with rheumatoid arthritis., PMID:35543863
High Titers of Low Affinity Antibodies in COVID-19 Patients Are Associated With Disease Severity., PMID:35493512
Hydrophilic rhodamine B-loaded / boronic acid-modified graphene oxide nanocomposite as a substitute of enzyme-labeled second antibody for ultrasensitive detection of antibodies., PMID:35490506
SARS-CoV2 wild type and mutant specific humoral and T cell immunity is superior after vaccination than after natural infection., PMID:35468147