Catalog No.
DXX04801
Expression system
Mammalian Cells
Species reactivity
Staphylococcus aureus
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
clfAClumping factor A
Concentration
3.62 mg/ml
Endotoxin level
Please contact with the lab for this information.
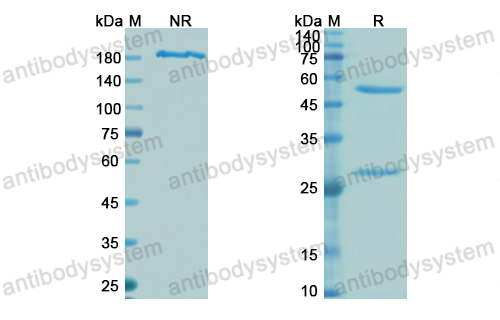
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q53653
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Aurexis, CAS: 521079-87-8
Clone ID
Tefibazumab
Drug evaluation: tefibazumab--a monoclonal antibody against staphylococcal infection, PMID: 17078388
Open-label, dose escalation study of the safety and pharmacokinetic profile of tefibazumab in healthy volunteers, PMID: 15728889
Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia, PMID: 16870768
Lessons from the Crystal Structure of the S. aureus Surface Protein Clumping Factor A in Complex With Tefibazumab, an Inhibiting Monoclonal Antibody, PMID: 27789272
Phase I dose escalation study to evaluate the safety and pharmacokinetic profile of tefibazumab in subjects with end-stage renal disease requiring hemodialysis, PMID: 17005843
A humanized monoclonal antibody targeting Staphylococcus aureus, PMID: 15576200
Registered and investigational drugs for the treatment of methicillin-resistant Staphylococcus aureus infection, PMID: 18221183
Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus, PMID: 16041045
The characteristics of pre-existing humoral imprint determine efficacy of S. aureus vaccines and support alternative vaccine approaches., PMID:38232694
Lessons from the Crystal Structure of the S. aureus Surface Protein Clumping Factor A in Complex With Tefibazumab, an Inhibiting Monoclonal Antibody., PMID:27789272
Registered and investigational drugs for the treatment of methicillin-resistant Staphylococcus aureus infection., PMID:18221183
Drug evaluation: tefibazumab--a monoclonal antibody against staphylococcal infection., PMID:17078388
Phase I dose escalation study to evaluate the safety and pharmacokinetic profile of tefibazumab in subjects with end-stage renal disease requiring hemodialysis., PMID:17005843
Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia., PMID:16870768
Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus., PMID:16041045
Open-label, dose escalation study of the safety and pharmacokinetic profile of tefibazumab in healthy volunteers., PMID:15728889
A humanized monoclonal antibody targeting Staphylococcus aureus., PMID:15576200