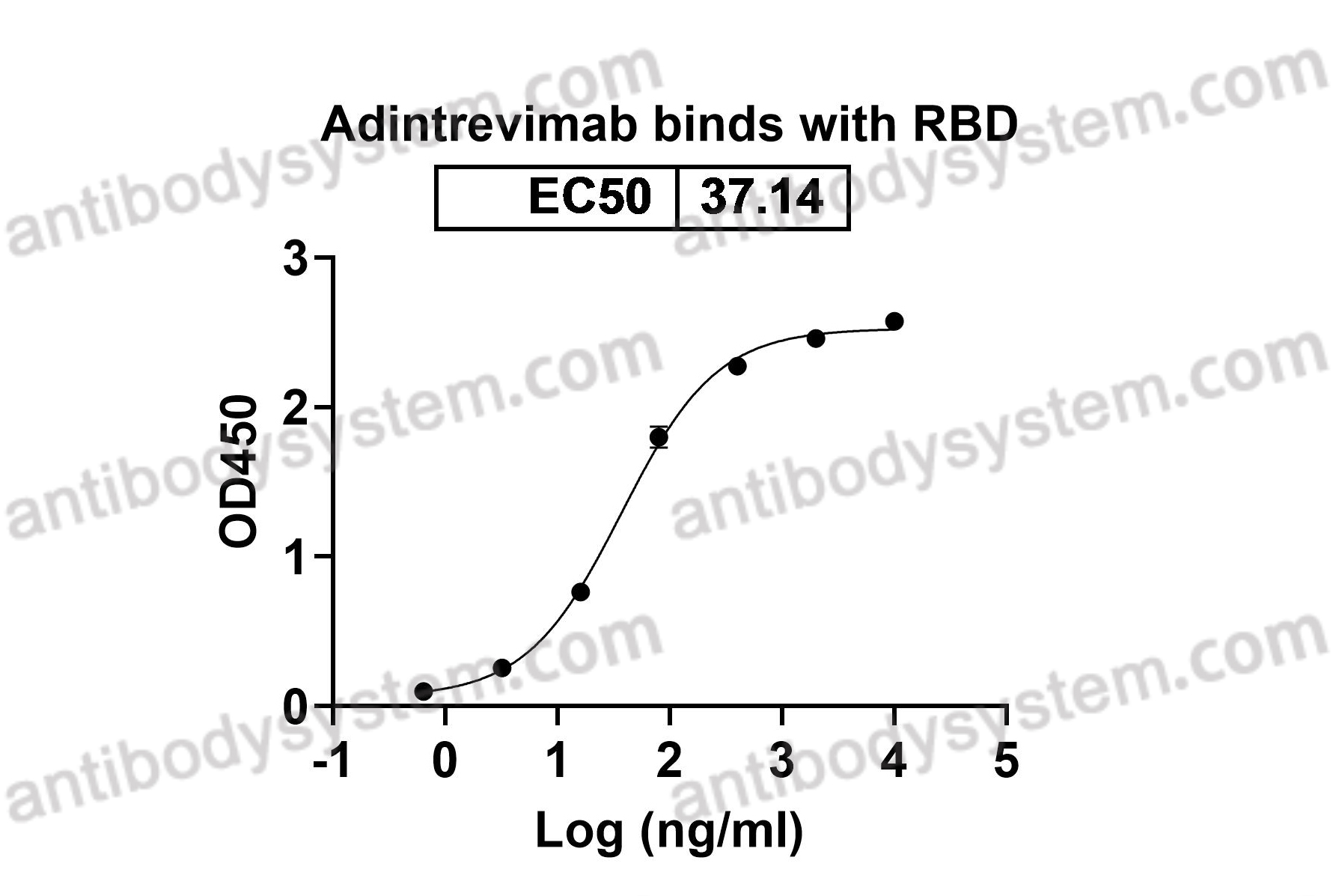
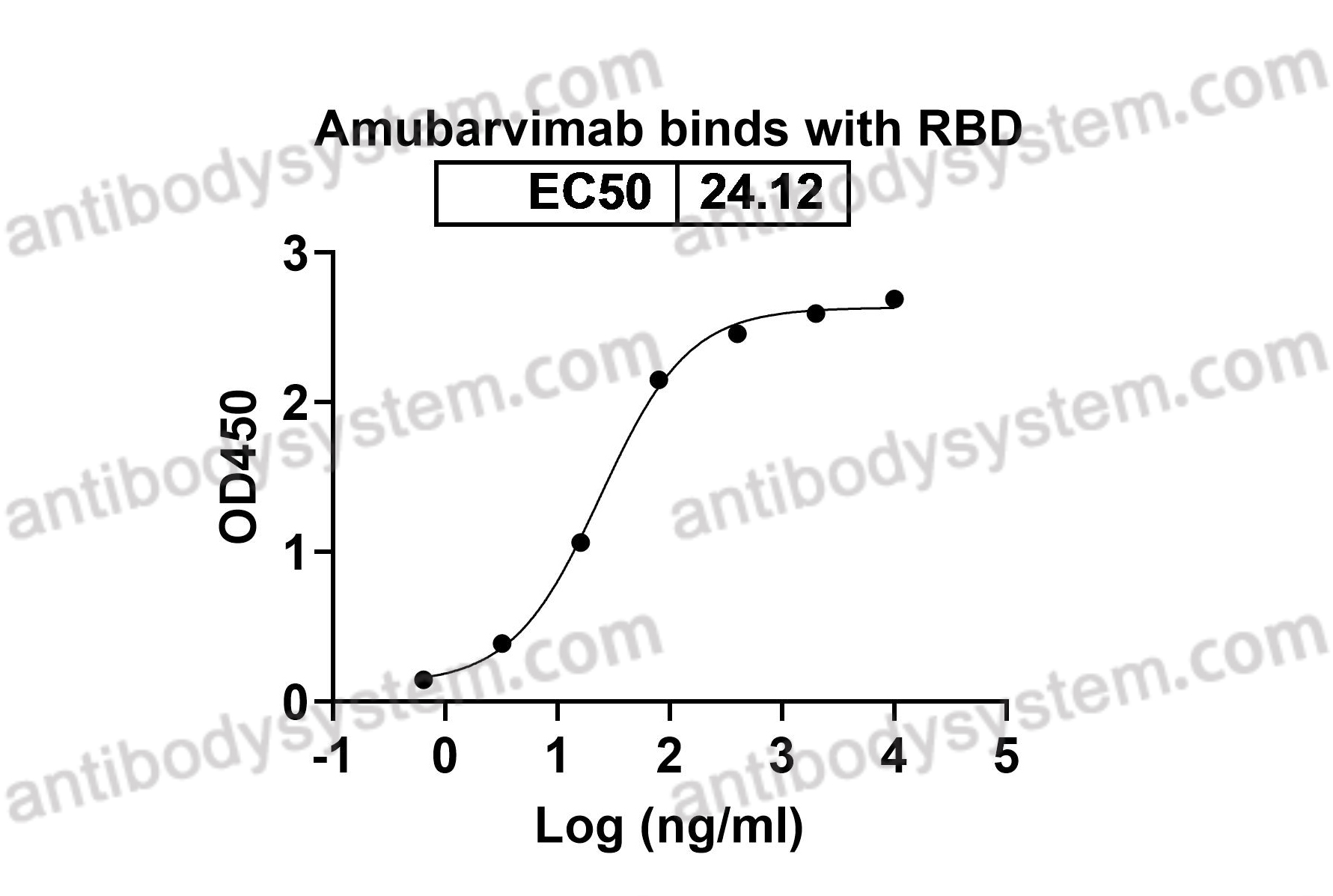
Catalog No.
EVV00302
Expression system
Mammalian Cells
Species
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Protein length
Arg319-Phe541
Predicted molecular weight
28.03 kDa
Nature
Recombinant
Concentration
1.74 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>90% as determined by SDS-PAGE.
Accession
P0DTC2
Applications
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Form
Liquid
Storage buffer
20mM PBS, pH7.4
Shipping
In general, proteins are provided as lyophilized powder/frozen liquid. They are shipped out with dry ice/blue ice unless customers require otherwise.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Alternative Names
Spike glycoprotein, S glycoprotein, E2, Peplomer protein, Spike protein S1, S, Receptor-Binding Domain, RBD, WT
Antibodies against SARS-CoV-2 non-structural protein 3 cross-react with human muscle cells and neuroglial cells. [EVV00302]
A combinatorial and computational Tandem approach towards a universal therapeutics against ACE2-mediated coronavirus infections., PMID:40520104
Demonstration and structural basis of a therapeutic DNA aptamer for SARS-CoV-2 spike protein detection., PMID:40516427
Applying Phage Display Technology to Obtain Specific Peptides Blocking Host-Virus Interactions., PMID:40515926
RBD Amplicon Sequencing and Evolutionary Analysis of SARS-CoV-2 Variants from Wastewater Samples., PMID:40515922
Exploring anti-SARS-CoV-2 natural products: dual-viral target inhibition by delphinidin and the anti-coronaviral efficacy of deapio platycodin D., PMID:40512442
Design of SARS-CoV-2 RBD immunogens to focus immune responses toward conserved coronavirus epitopes., PMID:40511920
Conformational Landscaping and Dynamic Mutational Profiling of Binding Interactions and Immune Escape for Broadly Neutralizing Class I Antibodies with SARS-CoV-2 Spike Protein: Distributed Binding Hotspot Networks Underlie Mechanism of Viral Resistance Against Existing Variants., PMID:40510568
Nonstabilized SARS-CoV-2 spike mRNA vaccination induces broadly neutralizing antibodies in nonhuman primates., PMID:40498855
Immune response induced by the recombinant novel coronavirus vaccine (Adenovirus type 5 vector) (Ad5-nCoV) in persons living with HIV (PLWH)., PMID:40498774
Detectable SARS-CoV-2 specific immune responses in recovered unvaccinated individuals 250 days post wild type infection., PMID:40498720
Determination of resilience of a panel of broadly neutralizing mAbs to emerging variants of SARS-CoV-2 generated using reverse genetics., PMID:40496810
Modulation of SARS-CoV-2 spike binding to ACE2 through conformational selection., PMID:40494932
Structural Analysis of the SARS-CoV-2 Spike N-Terminal Domain Across Wild-Type and Recent Variants: A Comparative Study., PMID:40485545
Genetic Variations in TLR2 and TLR4 Genes and Their Association With COVID-19 Severity and Inflammatory Markers in the Moroccan Population., PMID:40483559
Influence of Cholesterol on the Insertion and Interaction of SARS-CoV-2 Proteins with Lipid Membranes., PMID:40474679
GWAS identifies genetic loci for antibody response to SARS-CoV-2 vaccines in patients with systemic autoimmune diseases and healthy individuals., PMID:40463545
Exploring the standardized detection and sampling methods of human nasal SARS-CoV-2 RBD IgA., PMID:40463384
Phylogeny-driven design of broadly protective sarbecovirus receptor-binding domain nanoparticle vaccines., PMID:40463102
Exploring Virus Protein Domains: Detection and Evolutionary Insights., PMID:40455157
Allostery Links hACE2 Binding, Pan-variant Neutralization and Helical Extension in the SARS-CoV-2 Spike Protein., PMID:40447075
Multiplex ACE2-RBD binding inhibition assay: An integrated tool for assessing neutralizing antibodies to SARS-CoV-2 variants and protection against breakthrough infections., PMID:40447072
Development of bivalent RBD adapted COVID-19 vaccines for broad sarbecovirus immunity., PMID:40442110
COVID-19 vaccination and utility of booster dose: A community-based cross-sectional study., PMID:40440923
XBB.1.5 RBD-Based Bivalent Vaccines Induced Antibody Responses Against SARS-CoV-2 Variants in Mice., PMID:40432153
Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species., PMID:40431727
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year., PMID:40431652
Efficient Searches in Protein Sequence Space Through AI-Driven Iterative Learning., PMID:40429882
Rapid and Cost-Effective Diagnostic Blot Assays Based on the Use of Plant-Produced Recombinant Antigens: Lessons Learned from the SARS-CoV-2 RBD Antigen., PMID:40429644
Development of a self-assembling multimeric Bann-RBD fusion protein in Pichia pastoris as a potential COVID-19 vaccine candidate., PMID:40425664
Is SARS-CoV-2 Spike Evolution Being Retargeted at the N-Terminal Domain?, PMID:40415355
Maternal Vaccination and Protective Immunity Against SARS-CoV-2 Infection in Pups., PMID:40411252
Evaluation of broad-spectrum protection by novel mRNA vaccines against SARS-CoV-2 variants (Delta, Omicron-BA.5, XBB-EG.5) in the golden hamster model., PMID:40410742
Comparative study of humoral and cellular immunity against SARS-CoV-2 induced by different COVID-19 vaccine types: Insights into protection against wildtype, Delta and JN.1 omicron strains., PMID:40408899
Passive maternal immunity in children born to women with systemic autoimmune rheumatic disease - A case-control study., PMID:40408885
Food-derived bioactive pigment phycocyanobilin binds to SARS-CoV-2 spike protein both covalently and noncovalently affecting its conformation and functionality., PMID:40404003
Construction of a variable fragment (Fv)-immunoglobulin A (IgA) anti-receptor binding domain (RBD) SARS-CoV-2 library based on IgA from Indonesian COVID-19 survivors., PMID:40403817
Cross-reactive sarbecovirus antibodies induced by mosaic RBD nanoparticles., PMID:40402246
Mass Cytometry Analysis of High-Dimensional Single-Cell Immune Profiles in ZF2001-Vaccinated Patients Infected with SARS-CoV-2., PMID:40384795
Immunogenicity Evaluation of a SARS-CoV-2 BA.2 Subunit Vaccine Formulated with CpG 1826 plus alum Dual Adjuvant., PMID:40383947
An RBD-Fc mucosal vaccine provides variant-proof protection against SARS-CoV-2 in mice and hamsters., PMID:40383816
Analysis of the Total Immunoglobulin G (IgG) and Its Subclasses Over Time in Coronavirus Disease 2019-Recovered Patients and Its Association With Disease Severity: A Single-Center Prospective Cohort Study., PMID:40383689
Nanoparticle-supported, rapid, digital quantification of neutralizing antibodies against SARS-CoV-2 variants., PMID:40383030
Estimating population immunity to SARS-CoV-2 by random sampling from primary and secondary healthcare in Scotland, May 2024., PMID:40381379
Safety and immunogenicity of SARS-CoV-2 protein subunit recombinant vaccine (Indovac®) in healthy populations aged 18 years and above in Indonesia: A phase I, observer-blind, randomized, controlled study., PMID:40381203
A recombinant BCG with surface-displayed antigen induces humoral and cellular immune responses., PMID:40379714
Biophysics of SARS-CoV-2 spike protein's receptor-binding domain interaction with ACE2 and neutralizing antibodies: from computation to functional insights., PMID:40376405
Exploring Diverse Binding Mechanisms of Broadly Neutralizing Antibodies S309, S304, CYFN-1006 and VIR-7229 Targeting SARS-CoV-2 Spike Omicron Variants: Integrative Computational Modeling Reveals Balance of Evolutionary and Dynamic Adaptability in Shaping Molecular Determinants of Immune Escape., PMID:40376091
Electromagnetic waves destabilize the SARS-CoV-2 Spike protein and reduce SARS-CoV-2 Virus-Like particle (SC2-VLP) infectivity., PMID:40374718
Comparative performance of serum and plasma samples in SARS-CoV-2 serology and neutralization assays., PMID:40374015
Сomprehensive approach to comparative characteristics of clinical and laboratory parameters of the study in children - in 6 months after Covid-19 treatment and 6 months after Covid-19 vaccination., PMID:40367469