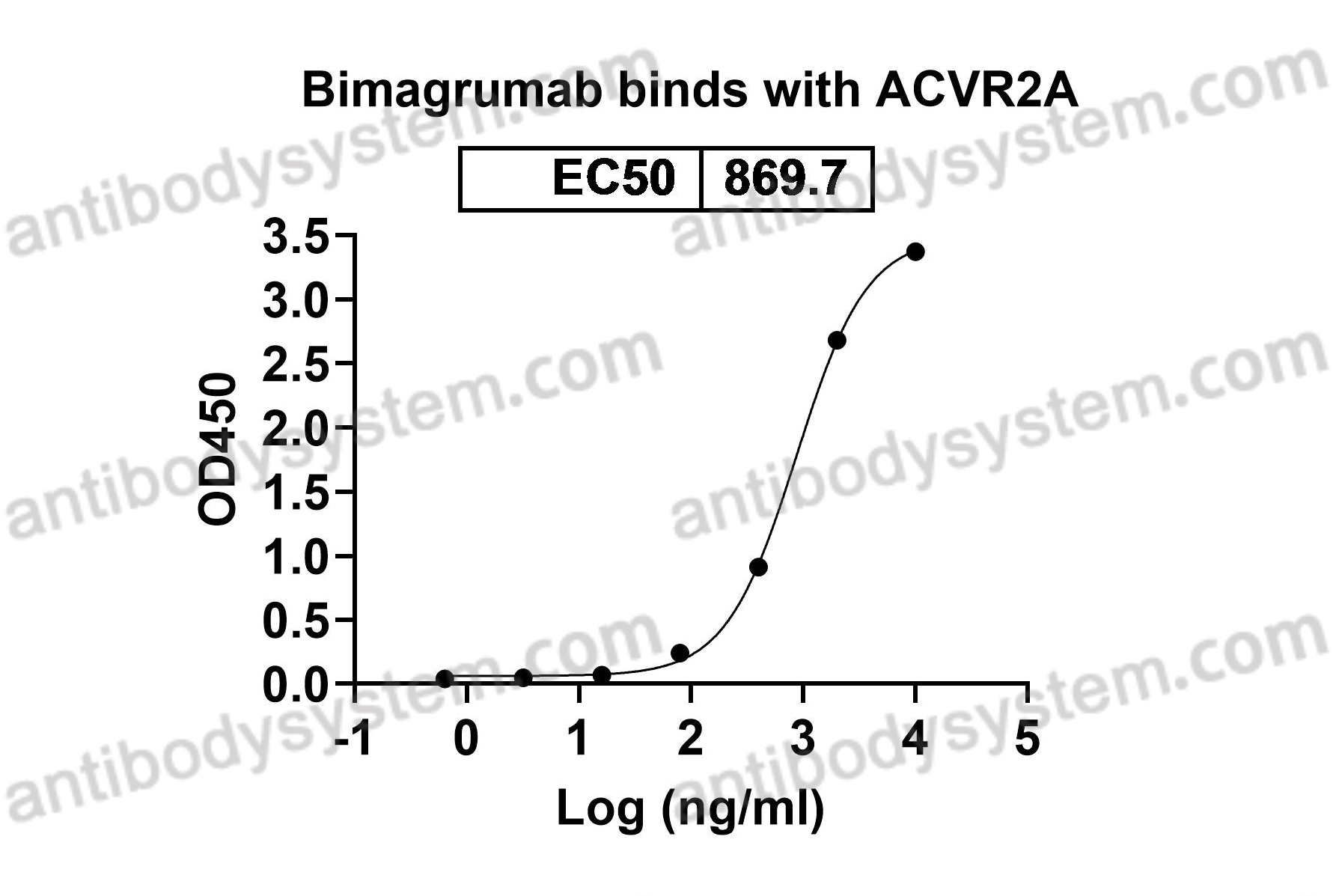
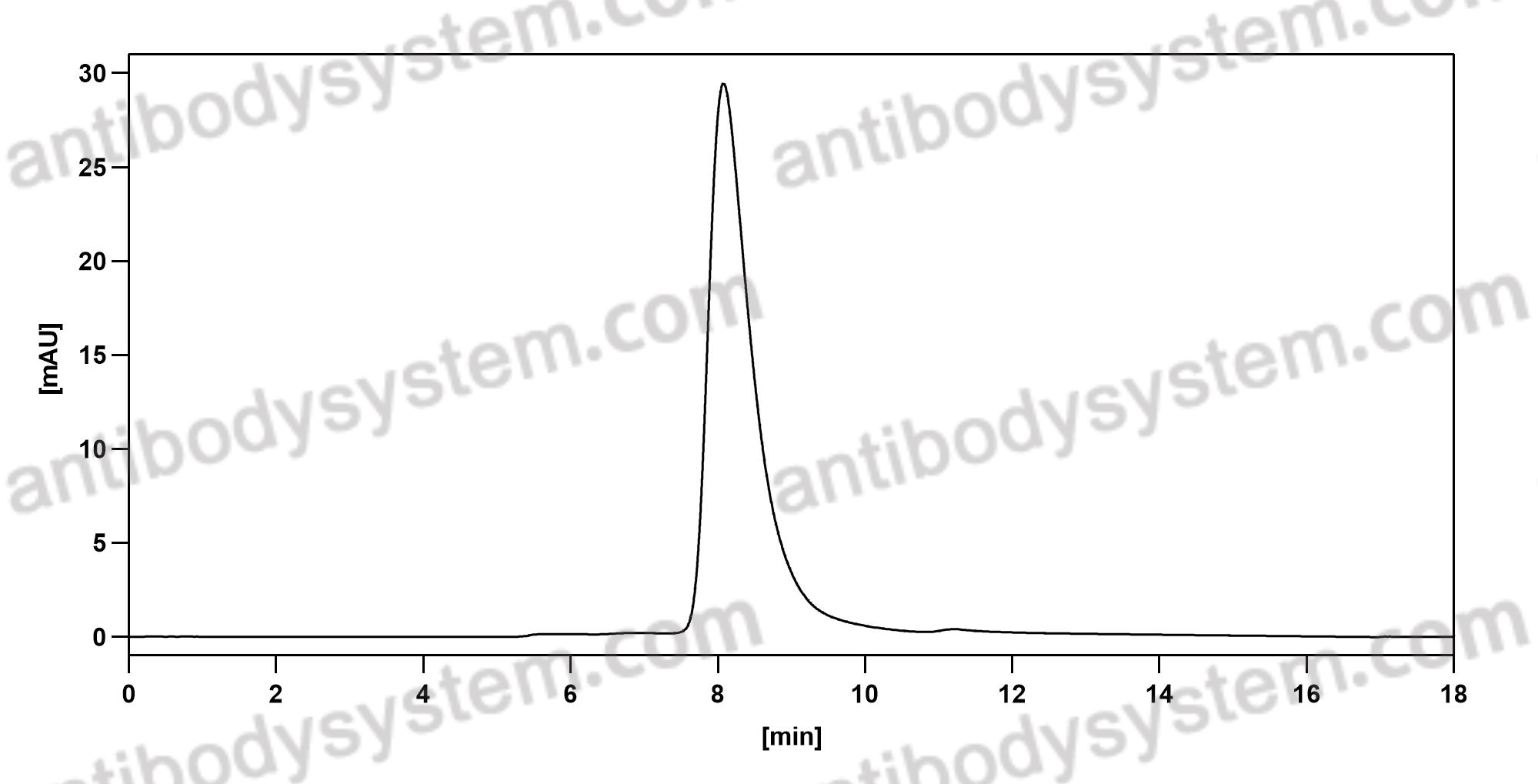
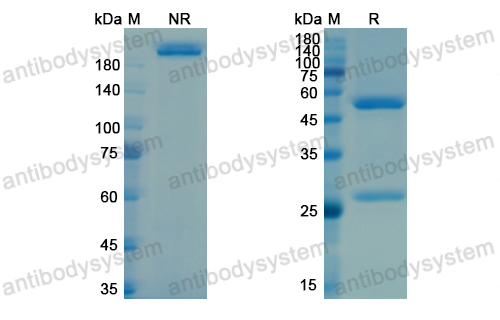
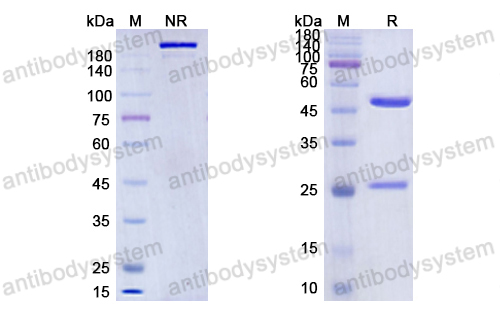
Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial, PMID: 33074327
Effect of Bimagrumab vs Placebo on Body Fat Mass Among Adults With Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial, PMID: 33439265
Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial, PMID: 31397289
A review on the treatment of sporadic inclusion body myositis with Bimagrumab and Alemtuzumab, PMID: 30238817
Effects of bimagrumab, an activin receptor type II inhibitor, on pituitary neurohormonal axes, PMID: 29566437
Safety and pharmacokinetics of bimagrumab in healthy older and obese adults with body composition changes in the older cohort, PMID: 33264516
Long-term safety and tolerability of bimagrumab (BYM338) in sporadic inclusion body myositis, PMID: 32690797
Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals, PMID: 28643356
Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen, PMID: 32992744
Effect of bimagrumab on thigh muscle volume and composition in men with casting-induced atrophy, PMID: 28905498
Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study, PMID: 28653345
Treatment of sporadic inclusion body myositis with bimagrumab, PMID: 25381300
Detection of the Human Anti-ActRII Antibody Bimagrumab in Serum by Means of Affinity Purification, Tryptic Digestion, and LC-HRMS, PMID: 29226558
Efficacy and Safety of Bimagrumab in Sporadic Inclusion Body Myositis: Long-term Extension of RESILIENT, PMID: 33597289
Does Activin Receptor Blockade by Bimagrumab (BYM338) Pose Detrimental Effects on Bone Healing in a Rat Fibula Osteotomy Model?, PMID: 27167138
[Neurology], PMID: 26946707
Activin Type II Receptor Blockade for Treatment of Muscle Depletion in Chronic Obstructive Pulmonary Disease. A Randomized Trial, PMID: 30095981
Therapeutic potential of muscle growth promoters in a stress urinary incontinence model, PMID: 32686522
Inclusion body myositis: update, PMID: 25215417
Diagnosis and Management of Immune-Mediated Myopathies, PMID: 28473041
Antibodies to watch in 2015, PMID: 25484055
Novel therapeutic options for cachexia and sarcopenia, PMID: 27382931
Novel investigational biologics for the treatment of cancer cachexia, PMID: 24707881
Mechanisms of skeletal muscle ageing; avenues for therapeutic intervention, PMID: 24880707
Blocking the activin IIB receptor with bimagrumab (BYM338) increases walking performance: A meta-analysis, PMID: 34405505
Muscle-bone interactions: From experimental models to the clinic? A critical update, PMID: 26506009
Antibodies to watch in 2014, PMID: 24284914
New Hope for Sarcopenia, PMID: 29148043
Ongoing developments in sporadic inclusion body myositis, PMID: 25399751
Development of two complementary LC-HRMS methods for analyzing sotatercept in dried blood spots for doping controls, PMID: 31218901
The COPD Pipeline XXXII, PMID: 28848893
No longer going to waste, PMID: 27153267
Antibodies to watch in 2016, PMID: 26651519
Reply to: New Hope for Sarcopenia, PMID: 29148045
[Late phase II/III study of BYM338 in patients with sporadic inclusion body myositis (RESILIENT): Japanese cohort data], PMID: 31761834
Endpoint choice for inclusion body myositis: a step too far?, PMID: 31397280
Challenges for Treatment Trials of Inclusion Body Myositis, PMID: 33597288
Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy, PMID: 29109273
ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti-cancer treatments, PMID: 27462398
An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy, PMID: 24298022
Emerging drugs affecting skeletal muscle function and mitochondrial biogenesis - Potential implications for sports drug testing programs, PMID: 26842585
Combined medical strategies for the management of type 2 diabetes mellitus and obesity in adults, PMID: 34165376
Commentary: Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy, PMID: 29726548
Sarcopenia Trials in Specific Diseases: Report by the International Conference on Frailty and Sarcopenia Research Task Force, PMID: 27883164
Bimagrumab and sarcopenia., PMID:40471389
Current and Emerging Parenteral and Peroral Medications for Weight Loss: A Narrative Review., PMID:40422561
Nutrition support whilst on glucagon-like peptide-1 based therapy. Is it necessary?, PMID:40401903
New drugs for the treatment of obesity: do we need approaches to preserve muscle mass?, PMID:40320499
A Generic Detection Method for the Doping Control Analysis of Fc-Fusion Proteins and Monoclonal Antibodies in Equine Plasma., PMID:40033065
The Effect of Anti-Activin Receptor Type IIA and Type IIB Antibody on Muscle, Bone and Blood in Healthy and Osteosarcopenic Mice., PMID:39887865
Efficacy and safety of pharmacological treatments in inclusion body myositis: a systematic review., PMID:39843353
The impact of weight loss on fat-free mass, muscle, bone and hematopoiesis health: Implications for emerging pharmacotherapies aiming at fat reduction and lean mass preservation., PMID:39481534
Simultaneous detection of myostatin-targeting monoclonal antibodies in dried blood spots and plasma using liquid chromatography-tandem mass spectrometry with field asymmetric ion mobility spectrometry., PMID:39405785
Bimagrumab: an investigational human monoclonal antibody against activin type II receptors for treating obesity., PMID:39385353
Effect of Bimagrumab on body composition: a systematic review and meta-analysis., PMID:39251484
An open-label 16-week study of liraglutide in adolescents with obesity post-sleeve gastrectomy., PMID:39103247
What is the pipeline for future medications for obesity?, PMID:38302593
Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism., PMID:38218536
An update on peptide-based therapies for type 2 diabetes and obesity., PMID:36608818
Pharmacokinetics and Pharmacodynamics of Bimagrumab (BYM338)., PMID:36527600
Antibody Therapies in Autoimmune Inflammatory Myopathies: Promising Treatment Options., PMID:35394612
Drugs for Treating Obesity., PMID:34783910
Understanding of sarcopenia: from definition to therapeutic strategies., PMID:34537916
Next Generation Antiobesity Medications: Setmelanotide, Semaglutide, Tirzepatide and Bimagrumab: What do They Mean for Clinical Practice?, PMID:34518444
Blocking the activin IIB receptor with bimagrumab (BYM338) increases walking performance: A meta-analysis., PMID:34405505
Combined medical strategies for the management of type 2 diabetes mellitus and obesity in adults., PMID:34165376
Bimagrumab to improve recovery after hip fracture in older adults: a multicentre, double-blind, randomised, parallel-group, placebo-controlled, phase 2a/b trial., PMID:36098133
Efficacy and Safety of Bimagrumab in Sporadic Inclusion Body Myositis: Long-term Extension of RESILIENT., PMID:33597289
Challenges for Treatment Trials of Inclusion Body Myositis., PMID:33597288
Effect of Bimagrumab vs Placebo on Body Fat Mass Among Adults With Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial., PMID:33439265
Safety and pharmacokinetics of bimagrumab in healthy older and obese adults with body composition changes in the older cohort., PMID:33264516
Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial., PMID:33074327
Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen., PMID:32992744
Long-term safety and tolerability of bimagrumab (BYM338) in sporadic inclusion body myositis., PMID:32690797
Therapeutic potential of muscle growth promoters in a stress urinary incontinence model., PMID:32686522
[Late phase II/III study of BYM338 in patients with sporadic inclusion body myositis (RESILIENT): Japanese cohort data]., PMID:31761834
Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial., PMID:31397289
Endpoint choice for inclusion body myositis: a step too far?, PMID:31397280
Development of two complementary LC-HRMS methods for analyzing sotatercept in dried blood spots for doping controls., PMID:31218901
A review on the treatment of sporadic inclusion body myositis with Bimagrumab and Alemtuzumab., PMID:30238817
Activin Type II Receptor Blockade for Treatment of Muscle Depletion in Chronic Obstructive Pulmonary Disease. A Randomized Trial., PMID:30095981
Commentary: Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy., PMID:29726548
Effects of bimagrumab, an activin receptor type II inhibitor, on pituitary neurohormonal axes., PMID:29566437
Detection of the Human Anti-ActRII Antibody Bimagrumab in Serum by Means of Affinity Purification, Tryptic Digestion, and LC-HRMS., PMID:29226558
Reply to: New Hope for Sarcopenia., PMID:29148045
New Hope for Sarcopenia., PMID:29148043
Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy., PMID:29109273
Effect of bimagrumab on thigh muscle volume and composition in men with casting-induced atrophy., PMID:28905498
The COPD Pipeline XXXII., PMID:28848893
Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study., PMID:28653345
Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals., PMID:28643356
Diagnosis and Management of Immune-Mediated Myopathies., PMID:28473041
Sarcopenia Trials in Specific Diseases: Report by the International Conference on Frailty and Sarcopenia Research Task Force., PMID:27883164
ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti-cancer treatments., PMID:27462398