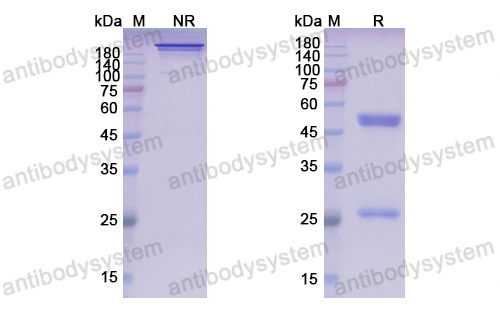
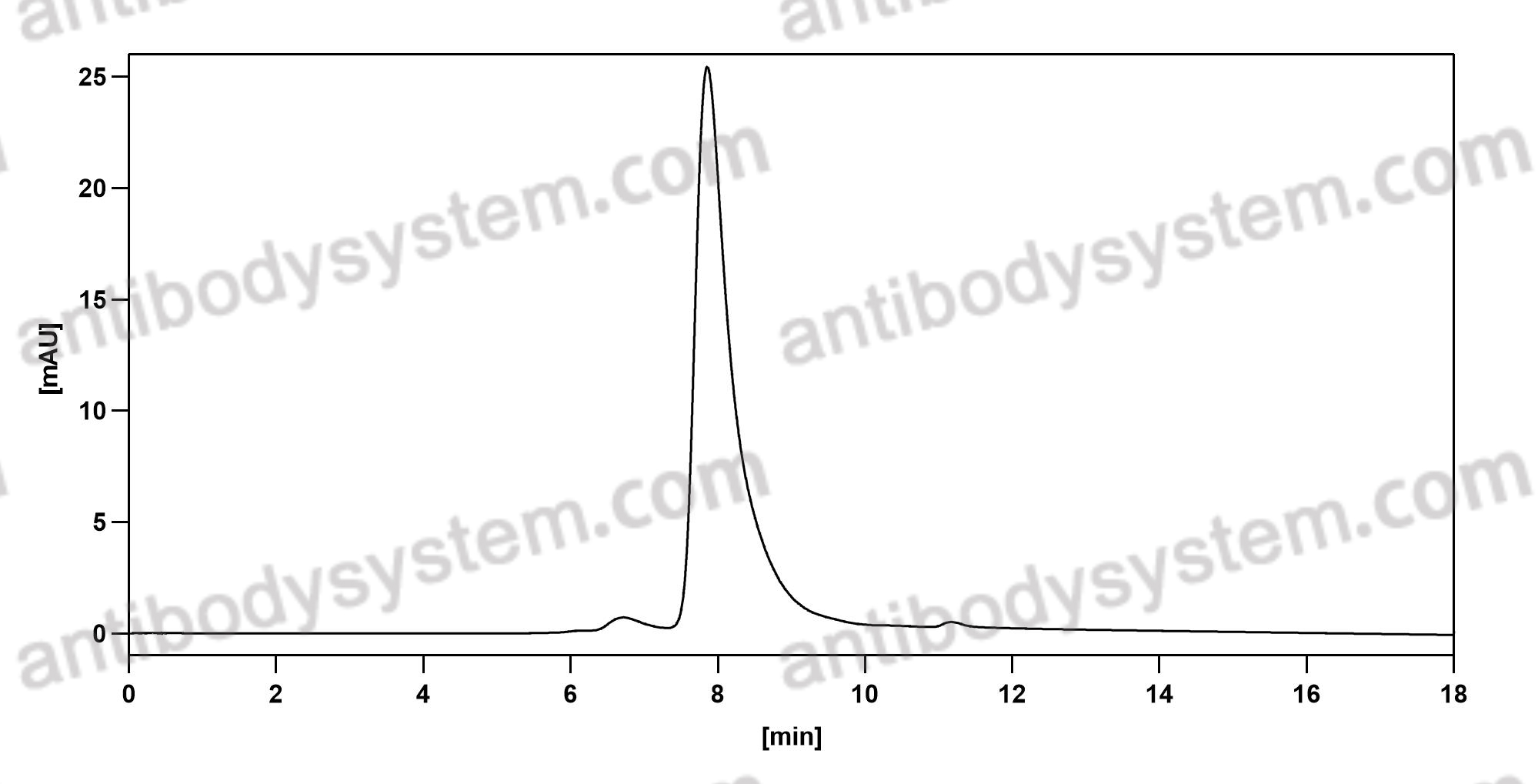
1,Characterization of zolbetuximab in pancreatic cancer models,OncoImmunology,Volume 8, 2019 - Issue 1 , https://doi.org/10.1080/2162402X.2018.1523096
2,Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer, J Hematol Oncol. 2017 May 12;10(1):105. doi: 10.1186/s13045-017-0473-4.
3,Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma,https://doi.org/10.1093/jjco/hyz068
A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study, PMID: 31240302
Characterization of zolbetuximab in pancreatic cancer models, PMID: 30546962
A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer, PMID: 29936063
Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer, PMID: 32410803
Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer, PMID: 28494772
Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma, PMID: 31087075
Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms, PMID: 23900716
FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma, PMID: 33610734
Patient-reported outcomes from the phase II FAST trial of zolbetuximab plus EOX compared to EOX alone as first-line treatment of patients with metastatic CLDN18.2+ gastroesophageal adenocarcinoma, PMID: 33755863
PLAC1 is essential for FGF7/FGFRIIIb-induced Akt-mediated cancer cell proliferation, PMID: 32499871
Claudin 18.2-a FAST-moving target in gastric cancer?, PMID: 33677015
Colitis-associated colorectal adenocarcinomas frequently express claudin 18 isoform 2: implications for claudin 18.2 monoclonal antibody therapy, PMID: 33590909
Antibody Improves Survival in Gastric Cancer, PMID: 27297550
Peptide microarrays enable rapid mimotope optimization for pharmacokinetic analysis of the novel therapeutic antibody IMAB362, PMID: 24497417
Navigating the clinical challenges of zolbetuximab in patients with claudin positive advanced gastric cancer., PMID:40466467
Guidelines of Onkopedia: What is new? Esophageal Cancer., PMID:40451170
Zolbetuximab-clzb: Targeting Claudin 18.2 in Advanced Gastric and Gastroesophageal Adenocarcinoma., PMID:40426305
Biomarker-Driven Approach to the Treatment of Metastatic Gastric or Gastroesophageal Adenocarcinoma., PMID:40341124
Claudin 18.2 Expression in Gastric Tumors and Other Tumor Types With Gastric Epithelium-like Differentiation., PMID:40295026
[Zolbetuximab in combination with chemotherapy in HER2-negative and CLDN 18.2-positive locally advanced unresectable or metastatic gastric or esogastric junction adenocarcinomas]., PMID:40280797
Computer-Aided Diagnostics Helps Accurately Determine Different Expression Levels of Claudin-18.2 in Gastric Cancer., PMID:40267901
Treatment Selection for Patients with HER2-Negative Metastatic Gastric Cancer Expressing Claudin 18.2 and PD-L1., PMID:40227631
CLDN18.2: a potential nanotherapeutic target for cholangiocarcinoma., PMID:40206086
Targeted Radionuclide Therapy of CLDN18.2-Positive Gastric Cancer with [131I]I-Zolbetuximab: An In Vitro and In Vivo Study., PMID:40200396
Two cases of protein-losing enteropathy induced by zolbetuximab in patients with unresectable advanced gastric cancer., PMID:40194315
Zolbetuximab-related gastritis: a case report of the patient with prolonged gastrointestinal symptoms., PMID:40178693
Future Landscape of Anti-Claudin 18.2 Antibodies in Gastric Adenocarcinoma., PMID:40136475
Advances and challenges in gastric cancer testing: the role of biomarkers., PMID:40126094
Claudin 18 (43-14A clone) expression in pancreatic ductal adenocarcinoma: Assessment of a potential clinical biomarker for zolbetuximab therapy., PMID:40117781
Expression of Claudin18.2 in metastatic lesions in peritoneum of gastric cancer., PMID:40115911
Evaluating the pharmacokinetics of zolbetuximab in gastric adenocarcinoma., PMID:40015974
Safety and Feasibility of Outpatient Zolbetuximab Administration in Community Cancer Care: A Mixed-methods Analysis., PMID:40010971
Monoclonal Antibodies in Metastatic Gastro-Esophageal Cancers: An Overview of the Latest Therapeutic Advances., PMID:39940858
Contemporary management of advanced gastric and gastroesophageal adenocarcinomas., PMID:39918299
Claudin 18.2: An attractive marker in pancreatic ductal adenocarcinoma., PMID:39850722
Claudin 18.2 Expression in 1404 Digestive Tract Adenocarcinomas Including 1175 Colorectal Carcinomas: Distinct Colorectal Carcinoma Subtypes Are Claudin 18.2 Positive., PMID:39826799
Zolbetuximab for Unresectable and Metastatic Gastric and Gastroesophageal Junction Adenocarcinoma: A Review of Literature., PMID:39759684
Zolbetuximab (Vyloy) for gastric and gastroesophageal carcinoma., PMID:39752728
Claudins in vulvar cancer - from epithelial barrier to potential tumor-agnostic cancer therapy., PMID:39722127
Efficacy and Safety of Zolbetuximab in Gastric Cancer., PMID:39699854
[A New Molecular Targeted Agent for Gastric Cancer-The Anti-Claudin 18.2 Antibody, Zolbetuximab]., PMID:39648123
Emerging targets in gastric and pancreatic cancer: Focus on claudin 18.2., PMID:39637967
First-line treatment with zolbetuximab plus CAPOX for ClDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: a cost-effectiveness analysis., PMID:39582898
[Claudine 18.2: A new therapeutic target in digestive cancers]., PMID:39516118
Immune checkpoint inhibitors in advanced gastroesophageal adenocarcinoma: a series of patient-level meta-analyses in different programmed death-ligand 1 subgroups., PMID:39426081
Expression of claudin 18.2 in poorly cohesive carcinoma and its association with clinicopathologic parameters in East Asian patients., PMID:39368365
Targeted and combination immunotherapies using biologics for gastric cancer: the state-of-the-art., PMID:39315517
Effect of antiemetics on zolbetuximab-induced gastric injury and emesis in ferrets., PMID:39313274
Claudin 1, 4, 6 and 18 isoform 2 as targets for the treatment of cancer (Review)., PMID:39301632
NEJM at ESMO - Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma., PMID:39282947
Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma., PMID:39282934
Targeting Claudin-18.2 for cancer therapy: updates from 2024 ASCO annual meeting., PMID:39183320
Variability in morphology and immunohistochemistry of Crohn's disease-associated small bowel neoplasms: implications of Claudin 18 and Cadherin 17 expression for tumor-targeted immunotherapies., PMID:39164422
[What's new in gastric cancer?]., PMID:39146748
Health-related quality of life in patients with CLDN18.2-positive, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma: results from the SPOTLIGHT and GLOW clinical trials., PMID:39146670
A novel highly sensitive inner filter effect-based fluorescence immunoassay with quantum dots for bioanalysis of zolbetuximab, a monoclonal antibody used for immunotherapy of gastric and gastroesophageal junction adenocarcinoma., PMID:39114008
Claudin-18.2 Immunohistochemical Evaluation in Gastric and Gastroesophageal Junction Adenocarcinomas to Direct Targeted Therapy: A Practical Approach., PMID:39098518
The impact of CLDN18.2 expression on effector cells mediating antibody-dependent cellular cytotoxicity in gastric cancer., PMID:39095563
Zolbetuximab on the SPOTLIGHT-a light spot?, PMID:38988087
Zolbetuximab: First Approval., PMID:38967717
Global prevalence of claudin 18 isoform 2 in tumors of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma., PMID:38954176
Preparation of Radiolabeled Zolbetuximab Targeting CLDN18.2 and Its Preliminary Evaluation for Potential Clinical Applications., PMID:38949095