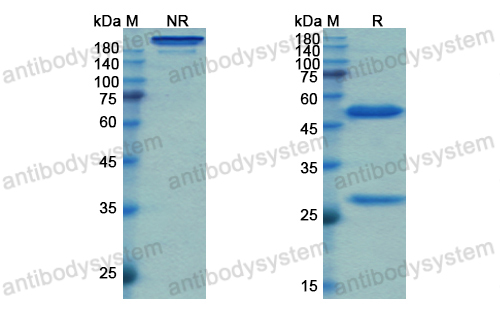
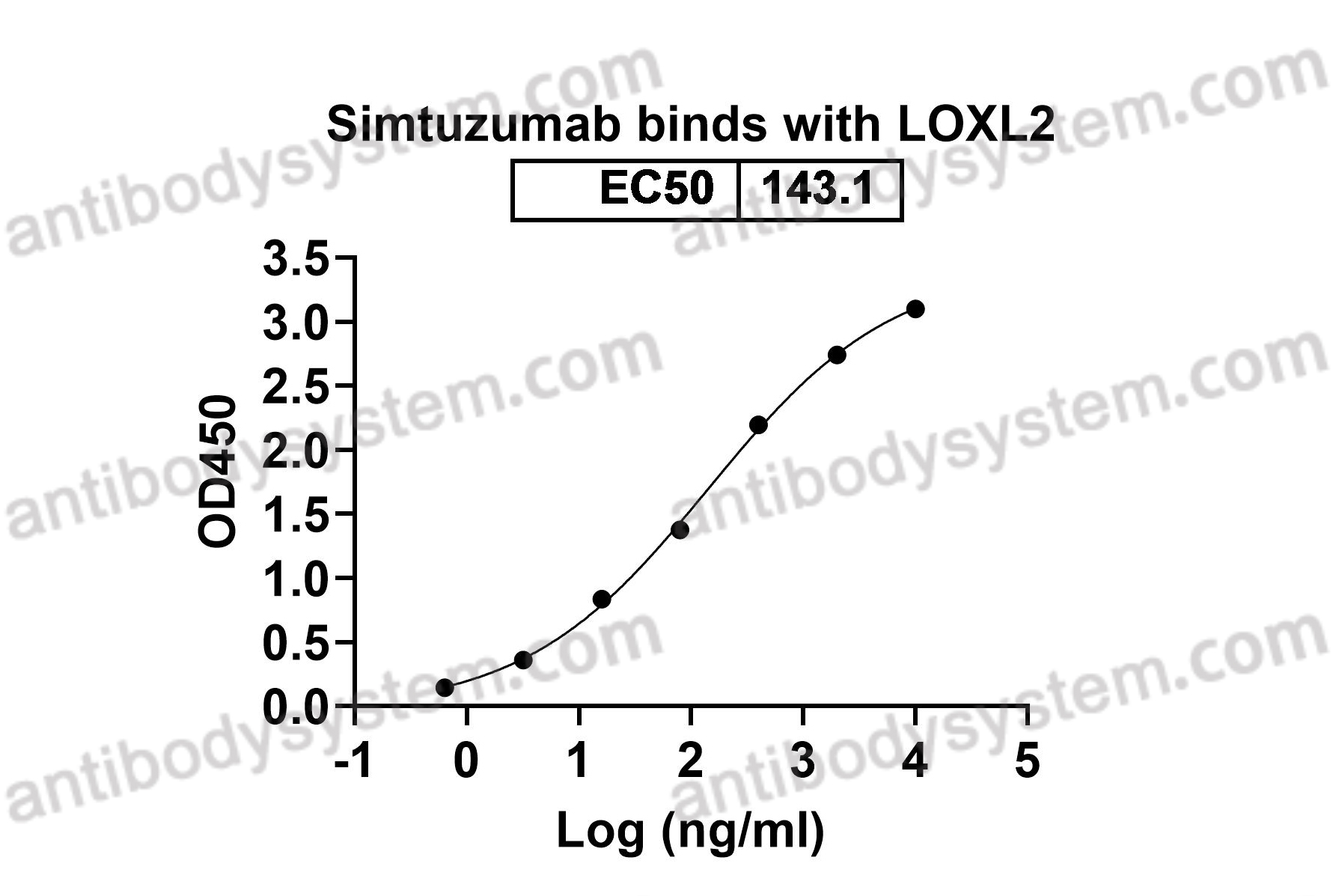
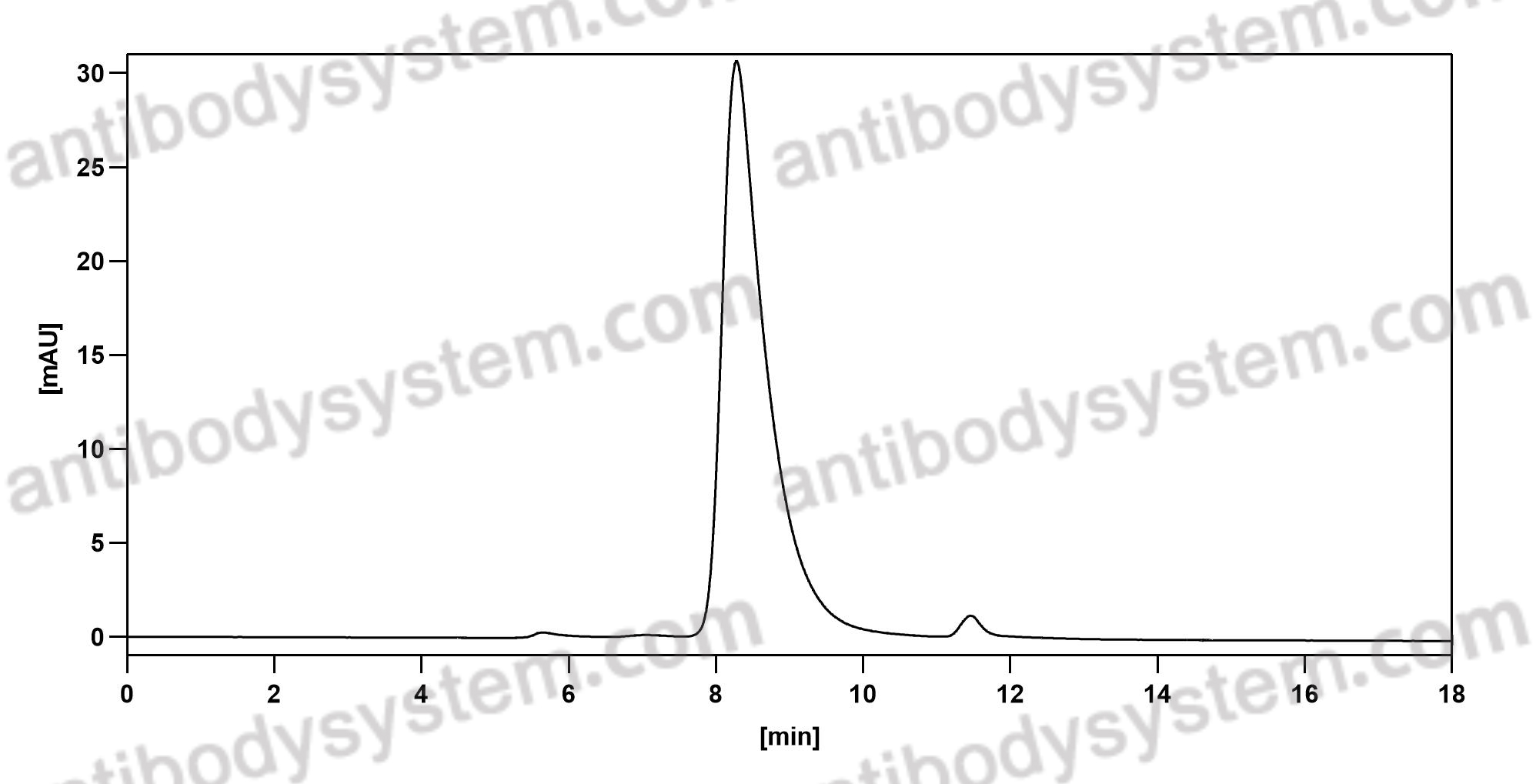
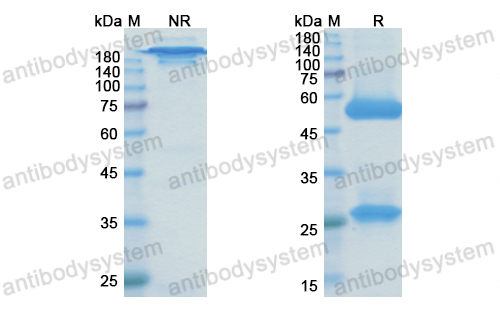
Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis, PMID: 29990488
Is This the Last Requiem for Simtuzumab?, PMID: 30303551
The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials, PMID: 30993748
Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease, PMID: 30153359
Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis, PMID: 32176358
The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial, PMID: 28892558
Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease, PMID: 27646933
Risk factors for disease progression in idiopathic pulmonary fibrosis, PMID: 31611341
Great expectations for simtuzumab in IPF fall short, PMID: 27939077
A phase 2 study of simtuzumab in patients with primary, post-polycythaemia vera or post-essential thrombocythaemia myelofibrosis, PMID: 28220932
Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: results of a 6-month open-label safety trial, PMID: 27232579
The Association of Histologic and Noninvasive Tests With Adverse Clinical and Patient-Reported Outcomes in Patients With Advanced Fibrosis Due to Nonalcoholic Steatohepatitis, PMID: 33307033
Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial, PMID: 27939076
A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma, PMID: 28246206
A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma, PMID: 28246207
A quick overview to the early phase clinical trials of Simtuzumab®: Are we loosing the most promising anti-fibrotic product?, PMID: 29055391
Emerging Treatments for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis, PMID: 29128056
Novel Pharmacotherapy Options for NASH, PMID: 27003143
Non-alcoholic fatty liver disease in 2015, PMID: 26085906
Therapy of Primary Sclerosing Cholangitis--Today and Tomorrow, PMID: 26641242
Inter- and Intra-individual Variation, and Limited Prognostic Utility, of Serum Alkaline Phosphatase in a Trial of Patients With Primary Sclerosing Cholangitis, PMID: 32707342
Emerging Therapies for Nonalcoholic Fatty Liver Disease, PMID: 27063275
[Treatment Options in Non-alcoholic Fatty Liver Disease], PMID: 28637104
Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib, PMID: 29377462
The 20% Rule of NASH Progression: The Natural History of Advanced Fibrosis and Cirrhosis Caused by NASH, PMID: 31520407
Methylation signatures in peripheral blood are associated with marked age acceleration and disease progression in patients with primary sclerosing cholangitis, PMID: 32039401
Spontaneous Fluctuations in Liver Biochemistries in Patients with Compensated NASH Cirrhosis: Implications for Drug Hepatotoxicity Monitoring, PMID: 31907854
Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: A hierarchical network meta-analysis, PMID: 33368954
Magnetic Resonance Elastography Shear Wave Velocity Correlates with Liver Fibrosis and Hepatic Venous Pressure Gradient in Adults with Advanced Liver Disease, PMID: 28480218
Controlling alpha for mixed effects models for repeated measures, PMID: 29461924
Statistical design for a confirmatory trial with a continuous predictive biomarker: A case study, PMID: 28522422
The role of LOX and LOXL2 in scar formation after glaucoma surgery, PMID: 23821193
Spatial transcriptomic validation of a biomimetic model of fibrosis enables re-evaluation of a therapeutic antibody targeting LOXL2., PMID:39173635
Serologic extracellular matrix remodeling markers are related to fibrosis stage and prognosis in a phase 2b trial of simtuzumab in patients with primary sclerosing cholangitis., PMID:38967589
Translational Studies Reveal the Divergent Effects of Simtuzumab Targeting LOXL2 in Idiopathic Pulmonary Fibrosis., PMID:38873180
Treatment of liver fibrosis: Past, current, and future., PMID:37397931
Liver stiffness thresholds to predict disease progression and clinical outcomes in bridging fibrosis and cirrhosis., PMID:36750244
Changes in the gut microbiome associated with liver stiffness improvement in nonalcoholic steatohepatitis., PMID:35601801
Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis., PMID:34662449
Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: A hierarchical network meta-analysis., PMID:33368954
The Association of Histologic and Noninvasive Tests With Adverse Clinical and Patient-Reported Outcomes in Patients With Advanced Fibrosis Due to Nonalcoholic Steatohepatitis., PMID:33307033
Inter- and Intra-individual Variation, and Limited Prognostic Utility, of Serum Alkaline Phosphatase in a Trial of Patients With Primary Sclerosing Cholangitis., PMID:32707342
Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis., PMID:32176358
Methylation signatures in peripheral blood are associated with marked age acceleration and disease progression in patients with primary sclerosing cholangitis., PMID:32039401
Spontaneous Fluctuations in Liver Biochemistries in Patients with Compensated NASH Cirrhosis: Implications for Drug Hepatotoxicity Monitoring., PMID:31907854
Risk factors for disease progression in idiopathic pulmonary fibrosis., PMID:31611341
The 20% Rule of NASH Progression: The Natural History of Advanced Fibrosis and Cirrhosis Caused by NASH., PMID:31520407
The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials., PMID:30993748
Is This the Last Requiem for Simtuzumab?, PMID:30303551
Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease., PMID:30153359
Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis., PMID:29990488
Controlling alpha for mixed effects models for repeated measures., PMID:29461924
Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib., PMID:29377462
Emerging Treatments for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis., PMID:29128056
A quick overview to the early phase clinical trials of Simtuzumab®: Are we loosing the most promising anti-fibrotic product?, PMID:29055391
The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial., PMID:28892558
[Treatment Options in Non-alcoholic Fatty Liver Disease]., PMID:28637104
Statistical design for a confirmatory trial with a continuous predictive biomarker: A case study., PMID:28522422
Magnetic Resonance Elastography Shear Wave Velocity Correlates with Liver Fibrosis and Hepatic Venous Pressure Gradient in Adults with Advanced Liver Disease., PMID:28480218
A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma., PMID:28246207
A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma., PMID:28246206
A phase 2 study of simtuzumab in patients with primary, post-polycythaemia vera or post-essential thrombocythaemia myelofibrosis., PMID:28220932
Great expectations for simtuzumab in IPF fall short., PMID:27939077
Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial., PMID:27939076
Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease., PMID:27646933
Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: results of a 6-month open-label safety trial., PMID:27232579
Emerging Therapies for Nonalcoholic Fatty Liver Disease., PMID:27063275
Novel Pharmacotherapy Options for NASH., PMID:27003143
Therapy of Primary Sclerosing Cholangitis--Today and Tomorrow., PMID:26641242
Non-alcoholic fatty liver disease in 2015., PMID:26085906
The role of LOX and LOXL2 in scar formation after glaucoma surgery., PMID:23821193