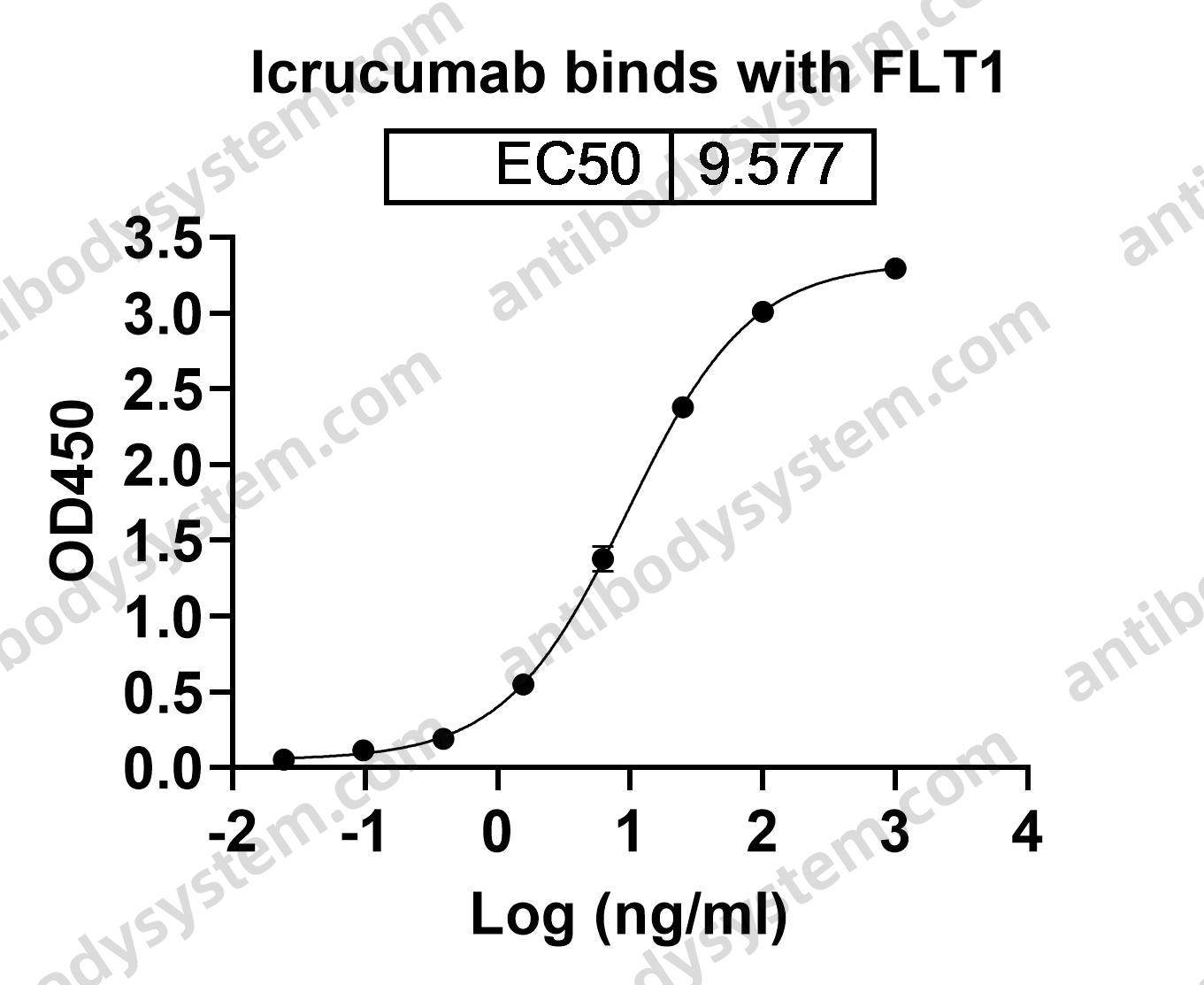
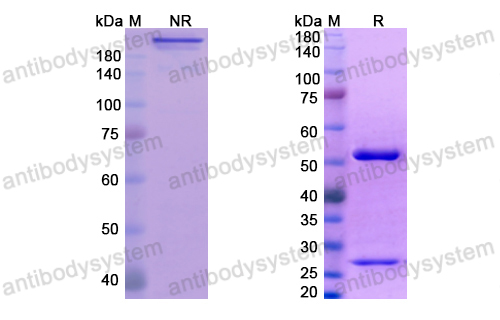
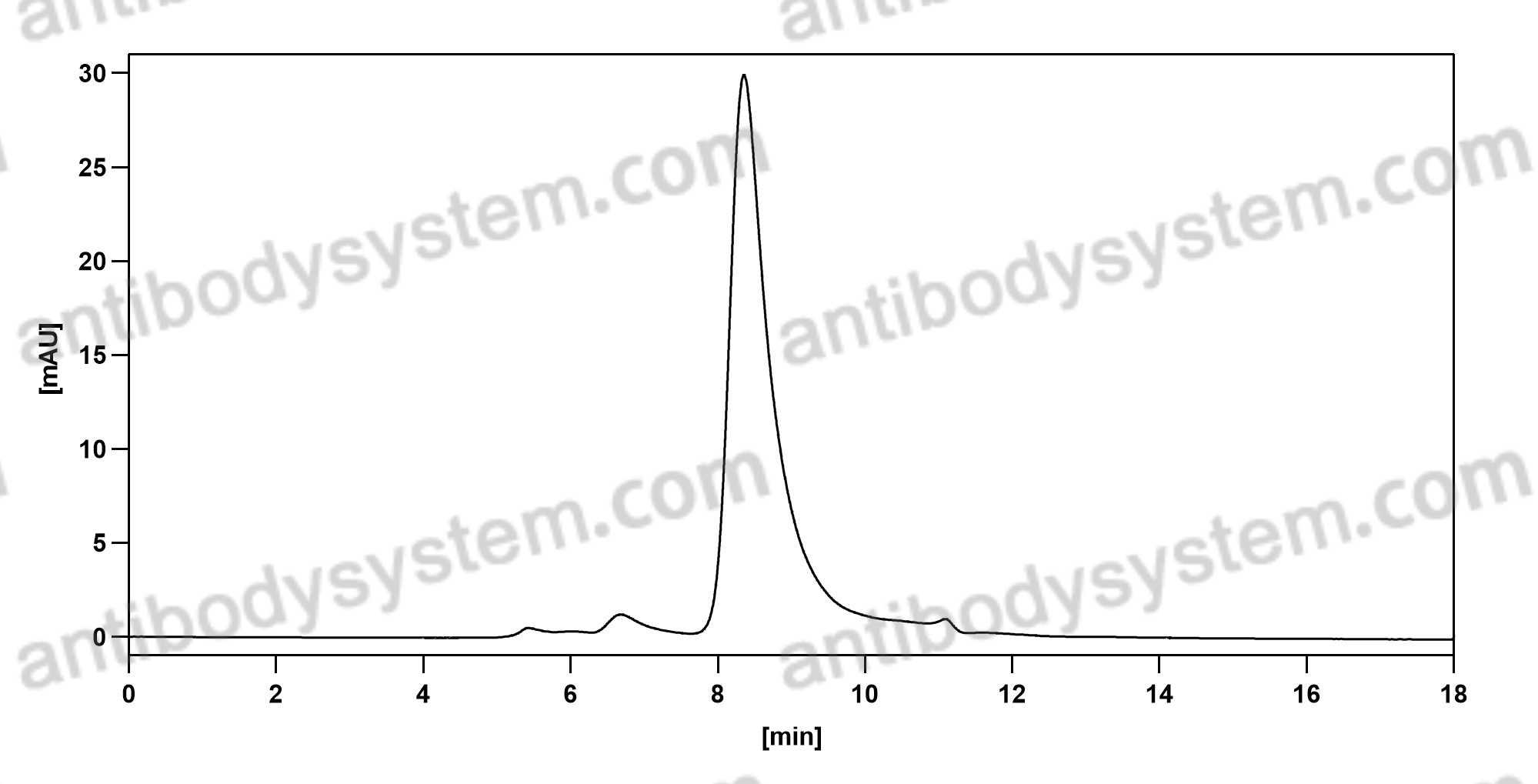
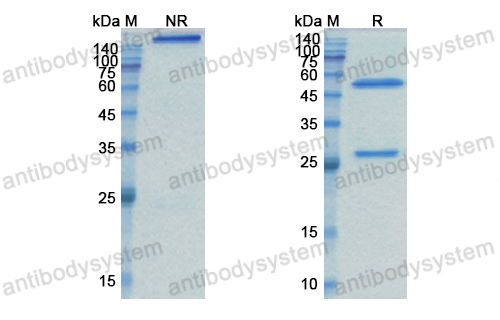
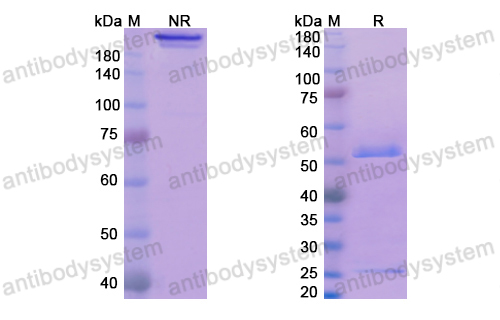
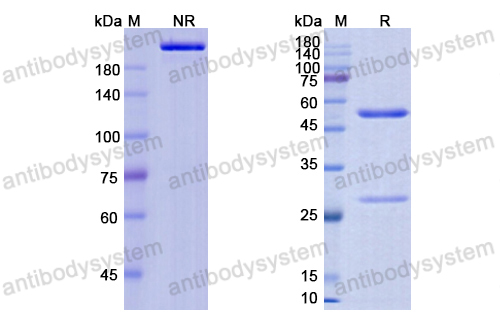
Catalog No.
DHD27401
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
FLT1, Vascular endothelial growth factor receptor 1, VEGFR1, Vascular permeability factor receptor, FRT, Tyrosine-protein kinase receptor FLT, VEGFR-1, FLT, FLT-1, Tyrosine-protein kinase FRT, Fms-like tyrosine kinase 1
Concentration
2.49 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P17948
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
18F1, IMC-18F1, LY3012212, CAS: 1024603-92-6
Clone ID
Icrucumab
Icrucumab, a fully human monoclonal antibody against the vascular endothelial growth factor receptor-1, in the treatment of patients with advanced solid malignancies: a Phase 1 study, PMID: 23903897
Randomized phase II study of modified FOLFOX-6 in combination with ramucirumab or icrucumab as second-line therapy in patients with metastatic colorectal cancer after disease progression on first-line irinotecan-based therapy, PMID: 27733377
Randomized Phase II Study of Ramucirumab or Icrucumab in Combination with Capecitabine in Patients with Previously Treated Locally Advanced or Metastatic Breast Cancer, PMID: 28220020
Docetaxel As Monotherapy or Combined With Ramucirumab or Icrucumab in Second-Line Treatment for Locally Advanced or Metastatic Urothelial Carcinoma: An Open-Label, Three-Arm, Randomized Controlled Phase II Trial, PMID: 26926681
Risk of hypertension with anti-VEGF monoclonal antibodies in cancer patients: a systematic review and meta-analysis of 105 phase II/III randomized controlled trials, PMID: 34229563
Metastasis and the Tumor Microenvironment: A Joint Metastasis Research Society-AACR Conference - Research on Metastasis: part 2, PMID: 21046523
Risk of hypertension with anti-VEGF monoclonal antibodies in cancer patients: a systematic review and meta-analysis of 105 phase II/III randomized controlled trials., PMID:34229563
Randomized Phase II Study of Ramucirumab or Icrucumab in Combination with Capecitabine in Patients with Previously Treated Locally Advanced or Metastatic Breast Cancer., PMID:28220020
Randomized phase II study of modified FOLFOX-6 in combination with ramucirumab or icrucumab as second-line therapy in patients with metastatic colorectal cancer after disease progression on first-line irinotecan-based therapy., PMID:27733377
Docetaxel As Monotherapy or Combined With Ramucirumab or Icrucumab in Second-Line Treatment for Locally Advanced or Metastatic Urothelial Carcinoma: An Open-Label, Three-Arm, Randomized Controlled Phase II Trial., PMID:26926681
Icrucumab, a fully human monoclonal antibody against the vascular endothelial growth factor receptor-1, in the treatment of patients with advanced solid malignancies: a Phase 1 study., PMID:23903897
Metastasis and the Tumor Microenvironment: A Joint Metastasis Research Society-AACR Conference - Research on Metastasis: part 2., PMID:21046523