Catalog No.
DHB95402
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-nd
Clonality
Monoclonal
Target
Interferon gamma, IFN-gamma, Immune interferon, IFNG
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
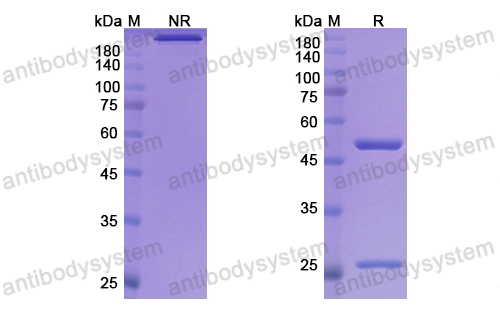
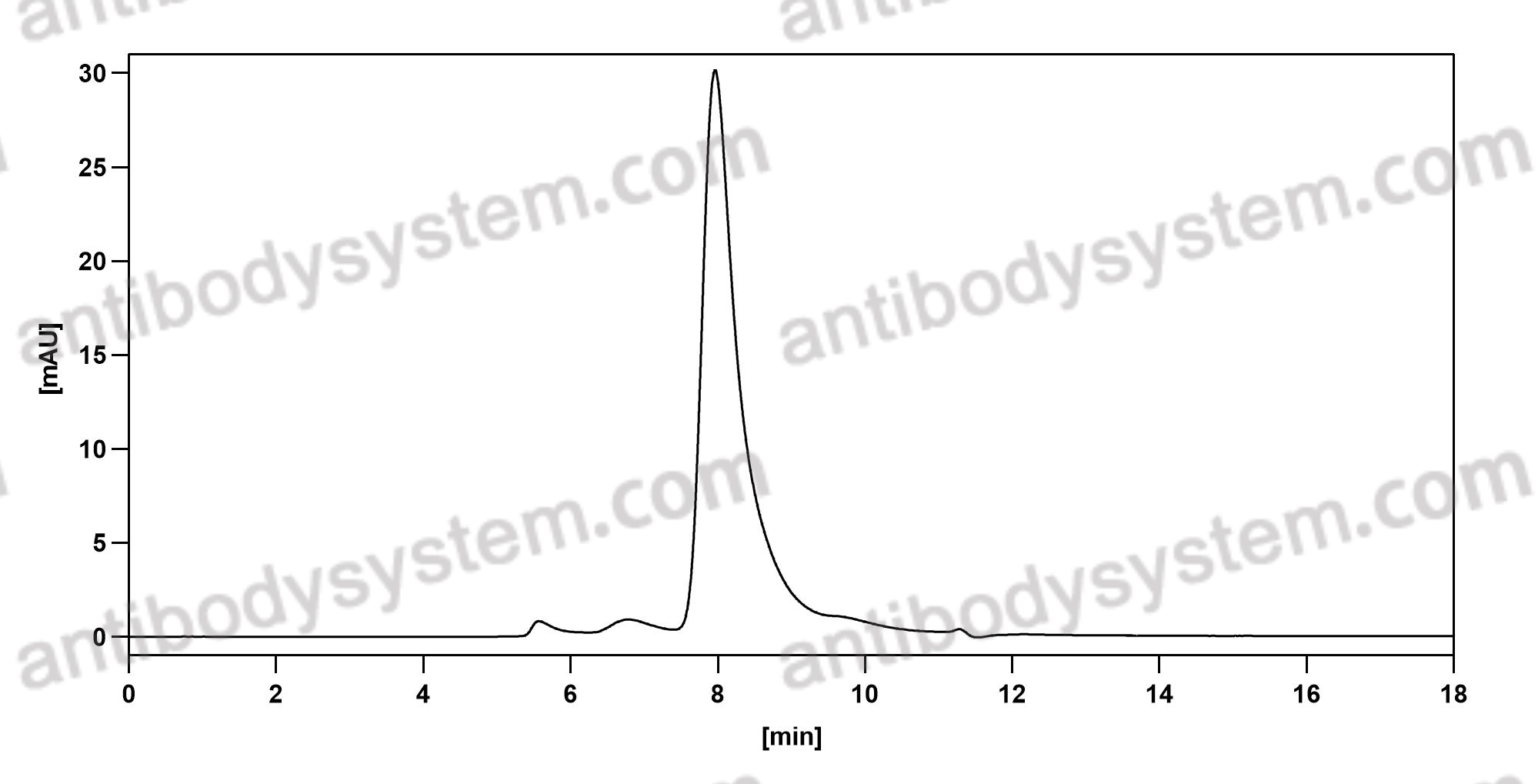
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01579
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
HuZAF, CAS: 326859-36-3
Clone ID
Fontolizumab
[Pharmacology of biologic medications], PMID: 24471295
A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn's disease, PMID: 16492717
Advances in therapeutic approaches to ulcerative colitis and Crohn's disease, PMID: 16313878
Efficacy and safety of interferon-gamma-targeted therapy in Crohn's disease: a systematic review and meta-analysis of randomized controlled trials, PMID: 23433962
Fontolizumab in moderate to severe Crohn's disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study, PMID: 19637334
Fontolizumab Protein Design Labs, PMID: 15912969
Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease, PMID: 16507585
Gateways to clinical trials, PMID: 16082427
Gateways to clinical trials, PMID: 17235418
How future tumor necrosis factor antagonists and other compounds will meet the remaining challenges in Crohn's disease, PMID: 15583528
Insights into gene modulation by therapeutic TNF and IFNgamma antibodies: TNF regulates IFNgamma production by T cells and TNF-regulated genes linked to psoriasis transcriptome, PMID: 17928893
Interfering with interferons in inflammatory bowel disease, PMID: 16849343
Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases, PMID: 27780712
Not all monoclonals are created equal - lessons from failed drug trials in Crohn's disease, PMID: 24913383
Novel therapeutic modalities in pediatric inflammatory bowel disease, PMID: 19070296
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID: 28765121
Overview of biologic therapy for Crohn's disease, PMID: 19591627
Potential immunotherapies for sarcoidosis, PMID: 29327613
Three-dimensional structures of a humanized anti-IFN-gamma Fab (HuZAF) in two crystal forms, PMID: 15388922
Tissue repair and ulcer/wound healing - Institut Pasteur Euroconference: molecular mechanisms, therapeutic targets and future directions, PMID: 15883915
Interfering With Inflammation: Heterogeneous Effects of Interferons in Graft-Versus-Host Disease of the Gastrointestinal Tract and Inflammatory Bowel Disease., PMID:34249014
Potential immunotherapies for sarcoidosis., PMID:29327613
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID:28765121
Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases., PMID:27780712
Not all monoclonals are created equal - lessons from failed drug trials in Crohn's disease., PMID:24913383
[Pharmacology of biologic medications]., PMID:24471295
Efficacy and safety of interferon-gamma-targeted therapy in Crohn's disease: a systematic review and meta-analysis of randomized controlled trials., PMID:23433962
Fontolizumab in moderate to severe Crohn's disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study., PMID:19637334
Overview of biologic therapy for Crohn's disease., PMID:19591627
Novel therapeutic modalities in pediatric inflammatory bowel disease., PMID:19070296
Insights into gene modulation by therapeutic TNF and IFNgamma antibodies: TNF regulates IFNgamma production by T cells and TNF-regulated genes linked to psoriasis transcriptome., PMID:17928893
Gateways to clinical trials., PMID:17235418
Interfering with interferons in inflammatory bowel disease., PMID:16849343
Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease., PMID:16507585
A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn's disease., PMID:16492717
Advances in therapeutic approaches to ulcerative colitis and Crohn's disease., PMID:16313878
Gateways to clinical trials., PMID:16082427
Fontolizumab Protein Design Labs., PMID:15912969
Tissue repair and ulcer/wound healing - Institut Pasteur Euroconference: molecular mechanisms, therapeutic targets and future directions., PMID:15883915
How future tumor necrosis factor antagonists and other compounds will meet the remaining challenges in Crohn's disease., PMID:15583528
Three-dimensional structures of a humanized anti-IFN-gamma Fab (HuZAF) in two crystal forms., PMID:15388922