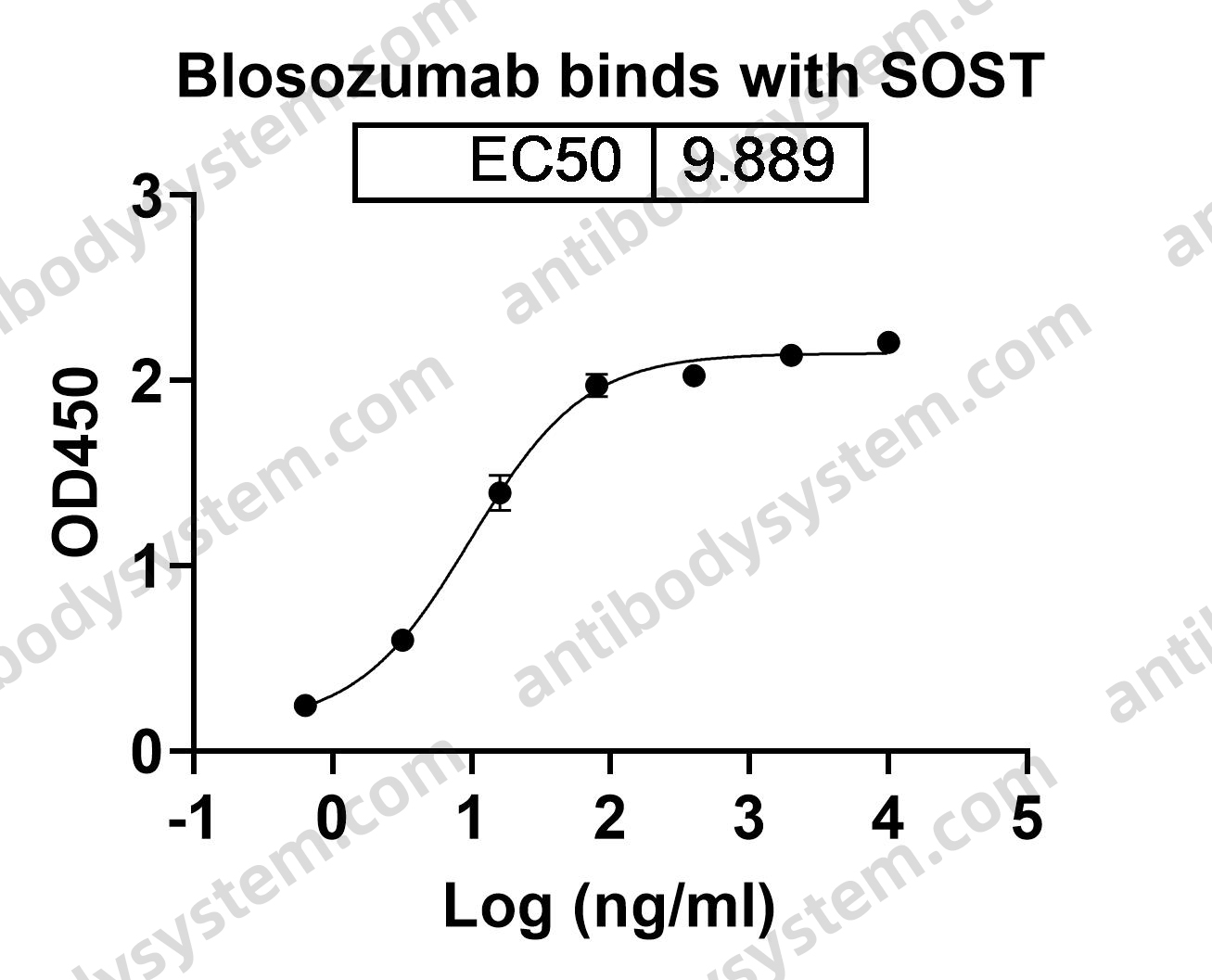
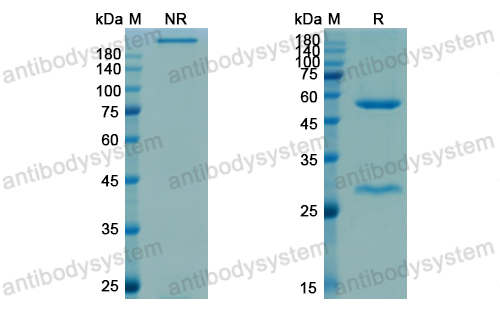
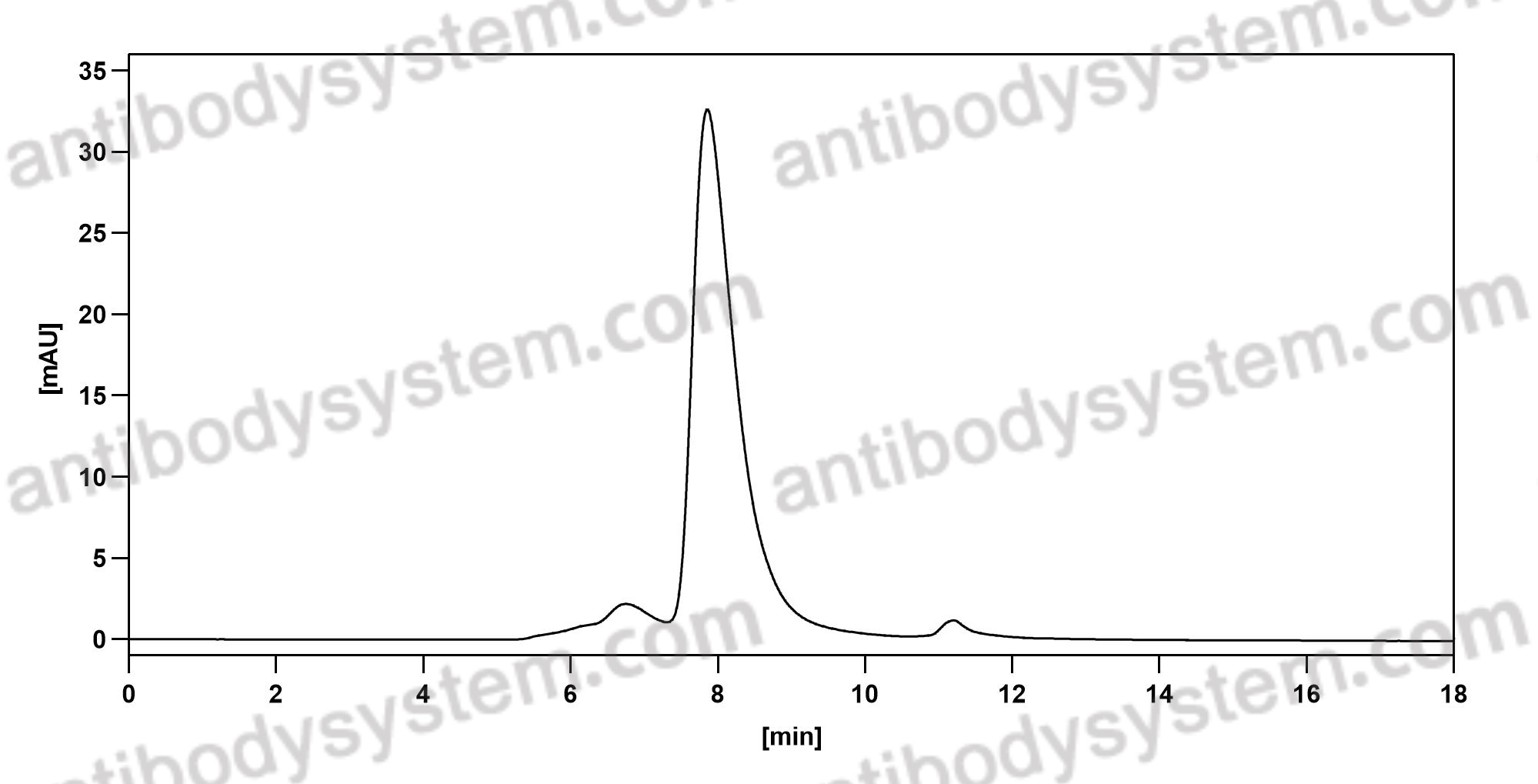
Targeting Sclerostin in Postmenopausal Osteoporosis: Focus on Romosozumab and Blosozumab, PMID: 28547661
Romosozumab and blosozumab: alternative drugs of mechanical strain-related stimulus toward a cure for osteoporosis, PMID: 25954248
Molecular genetics and targeted therapy of WNT-related human diseases (Review), PMID: 28731148
The Effect of Discontinuing Treatment With Blosozumab: Follow-up Results of a Phase 2 Randomized Clinical Trial in Postmenopausal Women With Low Bone Mineral Density, PMID: 25707611
Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women, PMID: 23996473
A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density, PMID: 25196993
Emerging drugs for osteoporosis, PMID: 24995794
Sclerostin and skeletal health, PMID: 25669441
Pharmacometrics and systems pharmacology for metabolic bone diseases, PMID: 30690761
Comparative efficacy between monoclonal antibodies and conventional drugs in postmenopausal women with osteoporosis: a network meta-analysis, PMID: 33302631
New insights into treatment of osteoporosis in postmenopausal women, PMID: 26557374
Biological agents in management of osteoporosis, PMID: 25204309
Anti-sclerostin antibodies: utility in treatment of osteoporosis, PMID: 24842796
Future directions for new medical entities in osteoporosis, PMID: 25432357
Sclerostin Inhibition in the Management of Osteoporosis, PMID: 27016922
Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis, PMID: 26082665
Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential, PMID: 28974988
Exploiting the WNT Signaling Pathway for Clinical Purposes, PMID: 28432596
Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases, PMID: 24688605
[Pharmacology of bone anabolic agents], PMID: 26529924
Clinical utility of anti-sclerostin antibodies, PMID: 28115281
[The canonical Wnt/β-catenin pathway: From the history of its discovery to clinical application], PMID: 28635854
Romosozumab: a novel bone anabolic treatment option for osteoporosis?, PMID: 31858345
Latest on Anabolic Agents for Osteoporosis Treatment., PMID:39448133
Rapid in vitro assessment of the immunogenicity potential of engineered antibody therapeutics through detection of CD4+ T cell interleukin-2 secretion., PMID:37682072
Blosozumab in the treatment of postmenopausal women with osteoporosis: a systematic review and meta-analysis., PMID:36367007
Sclerostin Inhibition: A Novel Target for the Treatment of Postmenopausal Osteoporosis., PMID:35264832
Efficacy and safety of anti-sclerostin antibodies in the treatment of osteoporosis: A meta-analysis and systematic review., PMID:34920938
Comparative efficacy between monoclonal antibodies and conventional drugs in postmenopausal women with osteoporosis: a network meta-analysis., PMID:33302631
Romosozumab: a novel bone anabolic treatment option for osteoporosis?, PMID:31858345
Pharmacometrics and systems pharmacology for metabolic bone diseases., PMID:30690761
Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential., PMID:28974988
Molecular genetics and targeted therapy of WNT-related human diseases (Review)., PMID:28731148
Targeting Sclerostin in Postmenopausal Osteoporosis: Focus on Romosozumab and Blosozumab., PMID:28547661
Exploiting the WNT Signaling Pathway for Clinical Purposes., PMID:28432596
Clinical utility of anti-sclerostin antibodies., PMID:28115281
Sclerostin Inhibition in the Management of Osteoporosis., PMID:27016922
[The canonical Wnt/β-catenin pathway: From the history of its discovery to clinical application]., PMID:28635854
New insights into treatment of osteoporosis in postmenopausal women., PMID:26557374
[Pharmacology of bone anabolic agents]., PMID:26529924
Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis., PMID:26082665
Romosozumab and blosozumab: alternative drugs of mechanical strain-related stimulus toward a cure for osteoporosis., PMID:25954248
The Effect of Discontinuing Treatment With Blosozumab: Follow-up Results of a Phase 2 Randomized Clinical Trial in Postmenopausal Women With Low Bone Mineral Density., PMID:25707611
Sclerostin and skeletal health., PMID:25669441
Future directions for new medical entities in osteoporosis., PMID:25432357
Biological agents in management of osteoporosis., PMID:25204309
A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density., PMID:25196993
Emerging drugs for osteoporosis., PMID:24995794
Anti-sclerostin antibodies: utility in treatment of osteoporosis., PMID:24842796
Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases., PMID:24688605
Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women., PMID:23996473